400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
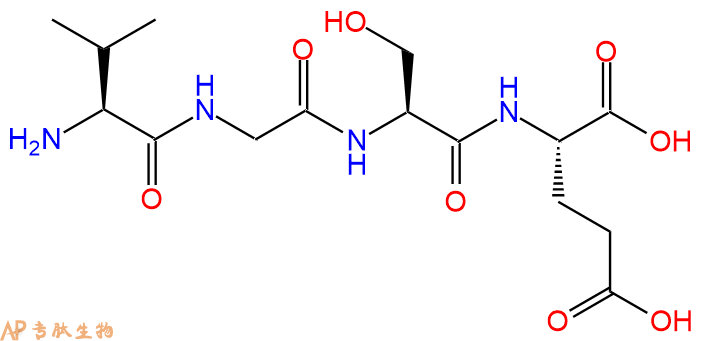
Eosinophilic chemotactic tetrapeptide also used as model peptide.
编号:191724
CAS号:61756-22-7
单字母:H2N-VGSE-OH
| 编号: | 191724 |
| 中文名称: | 四肽Eosinophilotactic Peptide |
| 英文名: | Eosinophilotactic Peptide |
| CAS号: | 61756-22-7 |
| 单字母: | H2N-VGSE-OH |
| 三字母: | H2N N端氨基:N-terminal amino group。在肽或多肽链中含有游离a-氨基的氨基酸一端。在表示氨基酸序列时,通常将N端放在肽链的左边。 -ValL-缬氨酸:valine。系统命名为(2S)-氨基-3-甲基丁酸。是编码氨基酸。是哺乳动物的必需氨基酸。符号:V,Val。在某些放线菌素如缬霉素中存在 D-缬氨酸。 -Gly甘氨酸:glycine。系统命名为 2-氨基乙酸。是编码氨基酸中没有旋光性的最简单的氨基酸,因具有甜味而得名。符号:G,Gly。 -SerL-丝氨酸:serine。系统命名为(2S)-氨基-3-羟基丙酸。是编码氨基酸。因可从蚕丝中获得而得名。符号:S,Ser。在丝原蛋白及某些抗菌素中含有 D-丝氨酸。 -GluL-谷氨酸:glutamic acid。系统命名为(2S)-氨基-戊二酸。是编码氨基酸。符号:E,Glu。D-谷氨酸存在于多种细菌的细胞壁和某些细菌杆菌肽中。 -OHC端羧基:C-terminal carboxyl group。在肽或多肽链中含有游离羧基的氨基酸一端。在表示氨基酸序列时,通常将C端放在肽链的右边。 |
| 氨基酸个数: | 4 |
| 分子式: | C15H26N4O8 |
| 平均分子量: | 390.39 |
| 精确分子量: | 390.18 |
| 等电点(PI): | 6.36 |
| pH=7.0时的净电荷数: | -0.02 |
| 酸性基团个数: | -1 |
| 碱性基团个数: | 亲水 |
| 平均亲水性: | 0.6 |
| 疏水性值: | -0.13 |
| 外观与性状: | 白色粉末状固体 |
| 闪点: | 0 M-1cm-1 |
| 消光系数: | - |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 嗜酸性四肽(Eosinophilotactic Tetrapeptides) |
Eosinophilic chemotactic tetrapeptide also used as model peptide.
H-Val-Gly-Ser-Glu-OH is a model system for the study of uptake and distribution of fluorescently labeled peptides. It is made up of four amino acids that are found in various proteins, and also have been shown to be potent inducers of inflammation. H-Val-Gly-Ser-Glu-OH has an acidic side chain and can be cleaved by proteases, which may account for its ability to enhance cell proliferation. H-Val-Gly-Ser-Glu-OH is localized to the epidermal growth factor receptor (EGFR) on the surface membrane of cells and shows chemotactic activity towards hl60 cells, which are human promyelocytic leukemia cells. This peptide also increases light emission from hl60 cells when cultured with monoclonal antibodies against EGFR.
Definition
Eosinophilotactic tetrapeptides exhibit peak in vitro chemotactic activity for human eosinophils and rapidly deactivate eosinophils to homologous and other stimuli at concentrations as low as 10 -10 M 1.
Related Peptides
The ECF-A acidic tetrapeptides Val-Gly-Ser-Glu, Ala-Gly-Ser-Glu and the analogue Val-Gly-Asp-Glu are selectively chemotactic for human eosinophils over a narrow dose range. Histamine abrogates the chemotactic properties of the individual tetrapeptides 2.
Discovery
The eosinophil chemotactic factor of anaphylaxis, ECF-A, was discovered in 1971 by Kay et al., as a mediator released during immediate-type hypersensitivity reactions in guinea pig and human lung slices 1.
Structural Characteristics
Two eosinophilotactic tetrapeptides of amino acid sequence Val-Gly-Ser-Glu and Ala-Gly-Ser-Glu were recovered from the extracts in 4-12% overall yield of the low molecular weight peak from Sephadex G-25. Purified eosinophil chemotactic factor of anaphylaxis and the synthetic tetrapeptides were maximally active in a chemotactic chamber, and the activity was dependent on both the NH2 terminal and the COOH-terminal residues. Both natural and synthetic peptides were preferentially chemotactic for eosinophils and rendered them unresponsive to a subsequent stimulus1.
Mode of Action
The chemotactic activity of the tetrapeptide is dependent on both the hydrophobic NH2-terminal residue, which interacts with a hydrophobic domain in the chemotactic receptor, and the highly-charged COOH-terminal residue which is presumed to initiate eosinophil movement by perturbing a polar domain in the same receptor. The spatial requirement for effective interaction with both domains in the receptor is revealed by the lower potency and activity of the condensed tripeptides lacking glycine. The 10-fold greater potency of NH2-terminal tripeptide compared to the amide derivatives of NH2-terminal amino acids in reversibly inhibiting the intact tetrapeptides suggests a role for serine in binding to a portion of the receptor, possibly by hydrogen bonding. The COOH-terminal substituent tripeptide irreversibly suppresses eosinophil chemotaxis by a cell-directed action possibly reflecting its capacity to perturb the polar domain; this effect, resembling de-activation, requires higher concentrations than needed for deactivation by the tetrapeptide 3.
Functions
ECF-A was discovered in 1971 as the mediator that is responsible for most of the eosinophil chemotactic activity released during anaphylactic reactions. Of the other mediators of immediate hypersensitivity, only histamine stimulates directed migration of eosinophils in vitro; however, its action is transient and lacks apparent in vivo chemotactic activity 4.
References
Goetzl EJ, Austen KF (1975). Purification and synthesis of eosinophilotactic tetrapeptides of human lung tissue: Identification as eosinophil chemotactic factor of anaphylaxis (leukocyte chemotaxis/leukocyte deactivation/ anaphylactic mediators/acidic peptides of lung). PNAS., 72(10):4123-4127.
Turnbull LW, Evans DP, Kay AB (1977). Human eosinophils, acidic tetrapeptides (ECF-A) and histamine. Interactions in vitro and in vivo. Immunology, 32(1):57-63.
Goetzl EJ, Austen KF (1976). Structural determinants of the eosinophil chemotactic activity of the acidic tetrapeptides of Eosinophil Chemotactic Factor of Anaphylaxis. J Exp Med., 144:1424-1437.
Goetzl EJ (1976). Modulation of human eosinophil polymorphonuclear leukocyte migration and function. Am J Pathol., 85(2):419-436.
| DOI | 名称 | |
|---|---|---|
| 10.1021/jf300497p | Identification of food-derived elastin peptide, prolyl-glycine (Pro-Gly), in human blood after ingestion of elastin hydrolysate | 下载 |
多肽H2N-Val-Gly-Ser-Glu-COOH的合成步骤:
1、合成CTC树脂:称取2.1g CTC Resin(如初始取代度约为0.84mmol/g)和2.12mmol Fmoc-Glu(OtBu)-OH于反应器中,加入适量DCM溶解氨基酸(需要注意,此时CTC树脂体积会增大好几倍,避免DCM溶液过少),再加入5.29mmol DIPEA(Mw:129.1,d:0.740g/ml),反应2-3小时后,可不抽滤溶液,直接加入1ml的HPLC级甲醇,封端半小时。依次用DMF洗涤2次,甲醇洗涤1次,DCM洗涤一次,甲醇洗涤一次,DCM洗涤一次,DMF洗涤2次(这里使用甲醇和DCM交替洗涤,是为了更好地去除其他溶质,有利于后续反应)。得到 Fmoc-Glu(OtBu)-CTC Resin。结构图如下:

2、脱Fmoc:加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Glu(OtBu)-CTC Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:

3、缩合:取5.29mmol Fmoc-Ser(tBu)-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入10.58mmol DIPEA,5.03mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Ser(tBu)-Glu(OtBu)-CTC Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:

4、依次循环步骤二、步骤三,依次得到
H2N-Ser(tBu)-Glu(OtBu)-CTC Resin
Fmoc-Gly-Ser(tBu)-Glu(OtBu)-CTC Resin
H2N-Gly-Ser(tBu)-Glu(OtBu)-CTC Resin
Fmoc-Val-Gly-Ser(tBu)-Glu(OtBu)-CTC Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Val-Gly-Ser(tBu)-Glu(OtBu)-CTC Resin,结构如下:
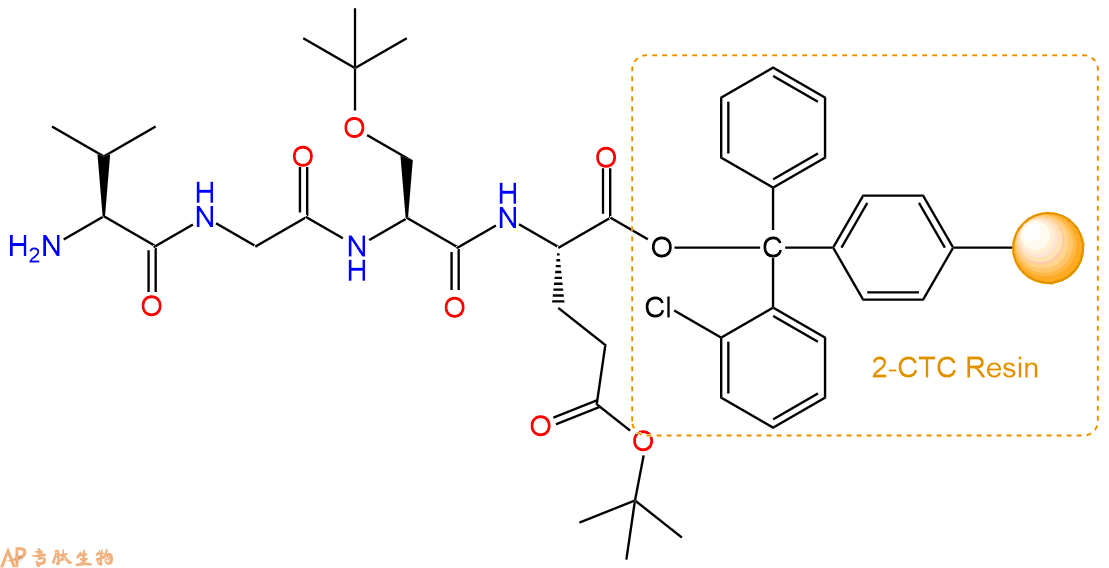
5、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品H2N-Val-Gly-Ser-Glu-COOH。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%、TFA:H2O=97.5%:2.5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





