400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研

FAM标记说明:
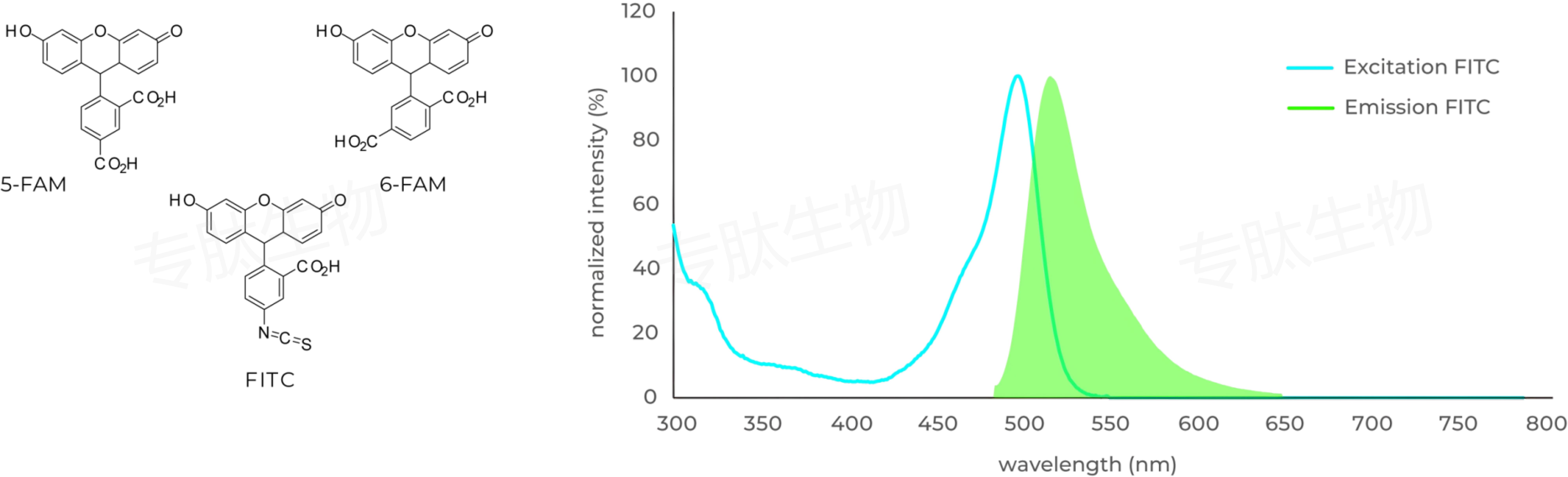
Carboxyfluorescein (FAM) is fluorophor with an excitation at 492 nm ▉ and emission of 517 nm ▉. Donors like FAM and 5FAM are often paired together with acceptors (CPQ2) for FRET experiments.
FAM标记肽的相关文献:
Porous Silicon Nanoparticle Delivery of Tandem Peptide Anti-Infectives for the Treatment of Pseudomonas aeruginosa Lung Infections.
Kwon, Ester J., et al. Advanced Materials 29.35 (2017).
Ultrasensitive tumor-penetrating nanosensors of protease activity.
Kwon, Ester J., Jaideep S. Dudani, and Sangeeta N. Bhatia. Nature Biomedical Engineering 1 (2017): 0054.
Seneca Valley Virus 3C pro Substrate Optimization Yields Efficient Substrates for Use in Peptide-Prodrug Therapy.
Miles, Linde A., et al. PloS One 10.6 (2015): e0129103.
The function of the milk-clotting enzymes bovine and camel chymosin studied by a fluorescence resonance energy transfer assay.
Jensen, Jesper Langholm, et al. Journal of Dairy Science 98.5 (2015): 2853-2860.
A comparison of modular PEG incorporation strategies for stabilization of peptide-siRNA nanocomplexes.
Lo, Justin H., et al. Bioconjugate Chemistry (2016).
Definition
Protein kinases are transferase that catalyze the phosphorylation of proteins by covalently attaching phosphate groups to them, using ATP as a phosphate donor. Reversible protein phosphorylation-dephosphorylation has a principal role in the regulation of essentially all cellular functions. Kinase/phosphatase substrates can be found grouped according to their kinase families. A single substrate can have a many number of modifications.
Discovery
Phoebus AL at the Rockefeller Institute identified phosphate in the protein Vitellin (phosvitin) in 1906 1 and by 1933 Fritz Lipmann had detected phosphoserine in Casein 2. In 1954 Eugene P. Kennedy described the first ‘enzymatic phosphorylation of proteins’ in a variety of normal and malignant tissues, he showed that the phosphorus of the phosphoprotein fraction undergoes a high rate of turnover, as measured by incorporation of 32P 3 .
Structural Characteristics
There are thousands of different kinds of proteins in any particular cell that are substrate for different kinases and phosphatases. Phosphorylation of any site on a given protein can change the structure, function or localization of that protein. Within a protein, phosphorylation can occur on several amino acids. Phosphorylation on serine is the most common, followed by threonine. Tyrosine phosphorylation is relatively rare. However, since tyrosine phosphorylated proteins are relatively easy to purify using antibodies, tyrosine phosphorylation sites are relatively well understood. Histidine and aspartate phosphorylation occurs in prokaryotes as part of two-component signaling and in some cases in eukaryotes in some signal transduction pathways 4. Phosphorylation of seryl or threonyl (and occasionally tyrosyl) residues triggers small conformational changes in these proteins that alter their biological properties.
Mode of Action
Phosphatase removes a phosphate group from its substrate by hydrolysing phosphoric acid monoesters into a phosphate ion and a molecule with a free hydroxyl group. Protein kinases are the effectors of phosphorylation and catalyse the transfer of a y-phosphate from ATP to specific amino acids on proteins. The addition of a phosphate (PO4) molecule to a polar R group of an amino acid residue can turn a hydrophobic portion of a protein into a polar and extremely hydrophilic portion of molecule. In this way it can introduce a conformational change in the structure of the protein via interaction with other hydrophobic and hydrophilic residues in the protein. Several protein kinases are important in cellular control (e.g. glycogen synthase kinase-3, acetyl CoA carboxylase kinase, tyrosine hydroxylase kinase and casein kinase-2), which are themselves controlled by allosteric effectors, phosphorylation, insulin and other growth factors, or by regulators. Protein phosphatase catalytic units are responsible for dephosphorylating many regulated proteins in the cytoplasm that are phosphorylated on serine and threonine residues. Some protein phosphatases are controlled by second messengers. PP-1(Protein phosphatase-1) is regulated by cyclic AMP in several ways that vary with the form of the enzyme and the tissue. It is inhibited by cyclic AMP through the phosphorylation of inhibitor-1 and its isoforms through the phosphorylation of targeting proteins such as the glycogen-binding subunit, and through allosteric inhibition by phosphorylase a. PP-2B (Protein phosphatase-2B) is activated by Ca2+ through the interaction of this second messenger with an integral Ca2+ -binding subunit, as well as calmodulin itself. Protein phosphorylation-dephosphorylation is the basis of a network of interlocking systems that allow hormones and other extracellular signals, acting through just a few second messengers, to coordinate biochemical functions 5.
Functions
Reversible phosphorylation of proteins is an important regulatory mechanism that occurs in living cells 6. Reversible phosphorylation results in a change in conformation the structure in many enzymes and receptors, causing them to become activated or deactivated.
Regulatory roles, the p53 protein is heavily regulated through phosphorylation sites, it has 18 different phosphorylation sites. Activation and phosphorylation of p53 can lead to cell cycle arrest, which can be reversed under some circumstances, or apoptotic cell death 7. In energy-requiring reactions, phosphorylation of Na+/K+-ATPase during the transport of sodium (Na+) and potassium(K+) ions across the cell membrane in osmoregulation to maintain homeostasis.
Enzyme regulation, phosphorylation of the enzyme GSK-3 by AKT (Protein kinase B) is a important regulation in insulin signaling pathway.
Protein-protein interaction, phosphorylation of the cytosolic components of NADPH oxidase, a large membrane-bound, multi-protein enzyme plays an important role in the regulation of protein-protein interactions of the enzyme.
Protein degradation, phosphorylation of some proteins causes them to be degraded by the ATP-dependent ubiquitin/proteasome pathway. These proteins become substrates for particular E3 ubiquitin ligases only when they are phosphorylated 8.
References
1. Levene PA, Alsberg CL (1906). The cleavage products of vitellin. J. Biol. Chem., 2(1): 127-133.
2. Lipmann FA, Levene PA (1932). Serinephosphoric acid obtained on hydrolysis of vitellinic acid. J. Biol. Chem., 98 (1):109-114.
3. Burnett G, Kennedy EP (1954). The enzymatic phosphorylation of proteins. J. Biol. Chem., 211(2):969–980.
4. Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD (2005). A simplified laminin nomenclature. Matrix Biol., 24(5):326-332.
5. Cohen P (1988). Protein Phosphorylation and Hormone Action. Proceedings of the Royal Society of London. Biological Sciences, 234(1275):115-144.
6. Barford D, Das AK, Egloff MP (1998). The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu Rev Biophys Biomol Struct., 27:133–164.
7. Ashcroft M, Kubbutat MH, Vousden KH (1999). Regulation of p53 function and stability by phosphorylation. Mol. Cell. Biol., 19(3):1751–1758.
8. Babior BM (1999). NADPH oxidase: an update. Blood, 93(5):1464–1476.





