400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研

P物质(Substance P)的定义
P物质(SP)是一种十一肽,在外周和中枢神经系统中都很丰富,它通常与经典的神经递质之一(最常见的是血清素(5-HT))共定位【1】。
Substance P (SP) an undecapeptide, is abundant both in the periphery and in the CNS, where it is usually co-localised with one of the classical neurotransmitters, most commonly serotonin (5- HT) 【1】.
P物质(Substance P)的相关肽
SP属于一种神经肽家族,称为速激肽,具有共同的C端序列:Phe-X-Gly-Leu-Met-NH2。三种最常见的速激肽是SP,神经激肽A(NKA)和神经激肽B(NKB);它们的生物学作用是通过指定为NK1,NK2和NK3的特定细胞表面受体介导的,SP是NK1受体的首选激动剂,NK2受体的NKA和NK3受体的NKB【2】。
SP belongs to a family of neuropeptides known as tachykinins that share the common C-terminal sequence: Phe-X-Gly-Leu-Met-NH2. The three most common tachykinins are SP, neurokinin A (NKA), and neurokinin B (NKB); their biologic actions are mediated through specific cell-surface receptors designated NK1, NK2, and NK3, with SP the preferred agonist for NK1 receptors, NKA for NK2 receptors, and NKB for NK3 receptors 【2】.
P物质(Substance P)的发现
SP最初是由von Euler和Gaddum于1931年发现的,是一种在体外引起肠道收缩的组织提取物;在随后的几十年中,进一步研究了其生物学作用和组织分布【3】。
SP was originally discovered in 1931 by von Euler and Gaddum as a tissue extract that caused intestinal contraction in vitro; its biologic actions and tissue distribution were further investigated over subsequent decades 【3】.
P物质(Substance P)的结构特征
SP是一种11个残基的神经肽,序列为Arg-Pro-Lys-Pro-Gln-Glin-Phe-Gly-Leu-Met-NH2)【4】。在一项研究中,将SP的C端和N端片段与亲本分子进行比较,以了解它们的能力:(a)收缩分离的豚鼠回肠,(b)诱导大鼠流涎,(C)激发单个猫背角神经元,以及(d)通过小鼠颅内注射诱导抓挠。与七肽一样小的C末端片段在所有测定系统上都是有效的SP激动剂。含有五个氨基酸或更少的C末端片段最多只有弱活性。N末端片段在分离的豚鼠回肠上完全无活性。然而,在大鼠唾液和中枢神经系统测定中,N末端片段能够产生弱的SP样活性【5】。获得的结果表明,虽然SP的羧基末端对于肽的支气管活性至关重要,但氨基末端肽(多达四个残基)的丢失实际上增强了支气管收缩剂对肽的反应。这种增强的部分原因似乎是SP和SP5-11的酶促降解差异所致。数据表明,二肽基氨肽酶切割SP可以增强其生物活性【6】。SP类似物:Senktide(琥珀酰-[Asp6,Me-Phe8]SP-(6-11))是NK-3(SP-N)受体的选择性类似物,其效力是SP的20-100倍,比驻留在肌肉细胞上的NK-1(SP-P)受体的选择性类似物强约1000倍【7】。在小鼠鞘内注射后,研究了五种SP类似物对神经激肽(NK)1受体激动剂如SP,physalaemin和(p-Glu6,Pro9)-SP(6-11)(septide)诱导的舔,咬和抓挠反应的影响。肽产生类似SP的行为反应,其效力约为D-Pro9类似物D-七肽的25倍。较低剂量的(D-Arg1,D-Pro2,4,D-Phe7,D-His9,Leu11)-SP比(D-Phe7,D-His9,Leu11)-SP(6-11)显着降低了Septide诱导的反应。相反,(D-Arg1,D-Pro2,4,D-Phe7,D-His9)-SP(0.5-1.0 nmol)和(D-Phe7,D-His9)-SP(6-11)(0.5-2.0 nmol)仅抑制SP诱导的行为反应,而不抑制藻毒素或七肽诱导的反应。这项研究的结果表明,NK-1受体激动剂不一定受到含有D-His 的SP类似物的相同程度的影响【8】。P物质[D-Arg1,D-Phe5,D-Trp7,9,Leu11]SP(SpD)和[Arg6,D-Trp7,9,NmePhe8]P物质的类似物可抑制神经肽刺激的Ca2+动员,酪氨酸磷酸化和ERK激活。至关重要的是,SpD和[Arg6,D-Trp7,9,NmePhe8]SP在体内和体外抑制SCLC细胞生长并刺激SCLC细胞凋亡。SP类似物最初被表征为“广谱神经肽拮抗剂”【9】。
SP is an 11-residue neuropeptide with the sequence Arg-Pro-Lys-Pro-Gln-Glin-Phe-Gly-Leu-Met-NH2) 【4】. In a study, the C- and N-terminal fragments of SP were compared to the parent molecule with respect to their ability to: (a) contract the isolated guinea pig ileum, (b) induce salivation in the rat, (c) excite single cat dorsal horn neurones, and (d) induce scratching by intracranial injections in mice. C-terminal fragments as small as the heptapeptide were potent SP agonists on all assay systems. C-terminal fragments containing five amino acids or less were, at most, only weakly active. N-terminal fragments were totally inactive on the isolated guinea pig ileum. On the rat salivation and central nervous system assays, however, N-terminal fragments were capable of weak SP-like activity 【5】. The results obtained, indicated that while the carboxy terminal of SP is essential for peptide bronchoactivity, loss of amino terminal peptides (up to four residues) actually enhances bronchoconstrictor responses to the peptide. Part of this enhancement appears to result from differences in the enzymatic degradation of SP and SP5-11. The data suggest that cleavage of SP by dipeptidyl aminopeptidases could enhance its bioactivity 【6】. SP analogs: Senktide (succinyl-[Asp6,Me-Phe8]SP-(6-11)), a selective analog for the NK-3 (SP-N) receptor, is 20-100 times more potent than SP and about 1000-fold more potent than the selective analogs for the NK-1 (SP-P) receptor, which resides on muscle cells 【7】. Effects of five SP analogs on the licking, biting and scratching response induced by neurokinin (NK) 1 receptor agonists such as SP, physalaemin and (p-Glu6,Pro9)-SP (6-11) (septide) were studied after intrathecal injections in mice. Peptide brought about a SP-like behavioral response, and was approximately 25 times more potent than the D-Pro9 analog, D-septide. Septide-induced response was significantly reduced by lower doses of (D-Arg1, D-Pro2,4, D-Phe7, D-His9, Leu11)-SP than (D- Phe7, D-His9, Leu11)-SP (6-11). In contrast, (D-Arg1, D-Pro2,4, D-Phe7, D-His9)-SP (0.5-1.0 nmol) and (D-Phe7, D-His9)-SP (6-11) (0.5-2.0 nmol) inhibited only SP-induced behavioral response, but not physalaemin- or septide-induced response. The results of this study indicate that NK-1 receptor agonists are not necessarily affected to a same degree by SP analogs containing D-His 8. Analogues of substance P, [D-Arg1,D-Phe5,D-Trp7,9,Leu11] SP (SpD) and [Arg6,D-Trp7,9,NmePhe8]substance P can inhibit neuropeptide-stimulated Ca2+ mobilization, tyrosine phosphorylation, and ERK activation . Crucially, SpD and [Arg6,D-Trp7,9,NmePhe8] SP inhibit SCLC cell growth in vivo and in vitro and stimulate SCLC cell apoptosis. SP analogues were characterized originally as "broad spectrum neuropeptide antagonists" 【9】.
P物质(Substance P)的作用方式
SP受体是一种G蛋白偶联受体,在许多方面类似于精神病学中其他经过充分研究的受体,特别是单胺受体2。SP与其受体的相互作用激活了Gq,Gq又激活了磷脂酶C,将磷脂酰肌醇二磷酸分解为肌醇三磷酸(IP3)和二酰基甘油(DAG)。IP3作用于肌浆网中的特定受体以释放细胞内的Ca2+,而DAG通过蛋白激酶C作用于打开质膜中的L型钙通道。细胞内[Ca2+]的升高诱导组织反应。与SP所见的一系列不同的行动,有许多治疗可能性【10】。
The SP receptor is a G protein-coupled receptor, in many respects similar to other well-studied receptors in psychiatry, particularly monoamine receptors 2. The interaction of SP with its receptor activates Gq, which in turn activates phospholipase C to break down phosphatidyl inositol bisphosphate into inositol trisphosphate (IP3) and diacylglycerol (DAG). IP3 acts on specific receptors in the sarcoplasmic reticulum to release intracellular stores of Ca2+, while DAG acts via protein kinase C to open L-type calcium channels in the plasma membrane. The rise in intracellular [Ca2+] induces the tissue response. With an array of actions as diverse as that seen with SP, there is scope for numerous therapeutic possibilities 【10】.
P物质(Substance P)的功能
在中枢神经系统中,SP与情绪障碍,焦虑,压力,强化,神经发生,神经毒性和疼痛的调节有关。在消化道中,SP和其他一些速激肽是调节运动活动,离子和液体分泌以及血管功能的神经递质【11,12】。
In the central nervous system, SP is associated with the regulation of mood disorders, anxiety, stress, reinforcement, neurogenesis, neurotoxicity and pain. In the digestive tract, SP, along with some other tachykinins, are neurotransmitters that regulate motor activity, secretion of ions and fluid, as well as vascular functions 【11,12】.
P物质(Substance P)的相关文献
1. Argyropoulos SV, Nutt DJ (2000). Substance P antagonists: novel agents in the treatment of depression. Expert Opin Investig Drugs, 9(8):1871-1875.
2. Book: Substance P and Related Tachykinins. Chapter 13: Neuropsychopharmacology: By Nadia MJ, Kramer MS.
3. Senba E, Tohyama M (1985). Origin and fine structure of substance P-containing nerve terminals in the facial nucleus of the rat:an immunohistochemical study. Exp Brain Res., 57(3):537-546.
4. Seidel MF, Tsalik J, Vetter H, Müller W (2007). Substance P in Rheumatic Diseases. Current Rheumatology Reviews, 3:17-30.
5. Piercey MF, Dobry PJ, Einspahr FJ, Schroeder LA, Masiques N (1982) Use of substance P fragments to differentiate substance P receptors of different tissues. Regulatory Peptides, 3(5-6):337-349.
6. Shore SA, Drazen JM (1988). Airway responses to substance P and substance P fragments in the guinea pig. Pulm Pharmacol., 1(3):113-118.
7. Hanani M, Chorev M, Gilon C, Selinger Z (1988). The actions of receptor-selective substance P analogs on myenteric neurons: an electrophysiological investigation. European journal of pharmacology, 153(2-3):247-253.
8. Sakurada T, Yamada T, Tan-no K, Manome Y, Sakurada S, Kisara K, Ohba M (1991). Differential effects of substance P analogs on neurokinin 1 receptor agonists in the mouse spinal cord. J Pharmacol Exp Ther., 259:205-210
9. MacKinnon AC, Waters C, Jodrell D, Haslett C, Sethi T (2001). Bombesin and Substance P Analogues Differentially Regulate G-protein Coupling to the Bombesin Receptor. J. Biol. Chem., 276(30):28083-28091..
10. Khawaja AM, Rogers DF (1996). Tachykinins: receptor to effector. Int J Biochem Cell Biol., 28(7):721-738.
11. Leeman SE, Mroz EA (1974). Substance P. Life Sci., 15(12):2033–2044.
12. Wiesenfeld-Hallin Z, Xu XJ (1993). The differential roles of substance P and neurokinin A in spinal cord hyperexcitability and neurogenic inflammation. Regul Pept., 46(1-2):165-173
甲基化修饰多肽
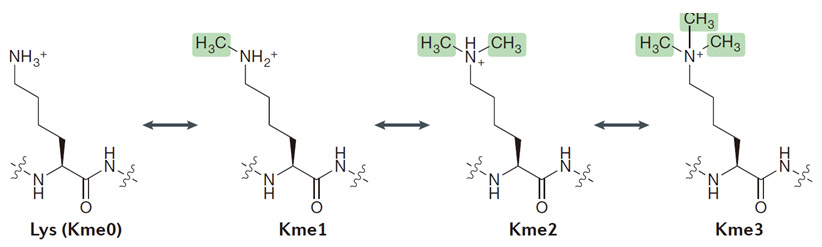
也叫甲基化标记多肽,甲基化修饰是在其他常见的翻译后修饰(PTMs)中生物和物理化学特性方面非常突出的一种修饰手段。几乎参与细胞所有的生命活动过程,发挥着重要的调控作用,蛋白质在甲基转移酶的催化下将甲基转移至特定的氨基酸残基上共价结合的过程。甲基化是一种可逆的修饰过程,由去甲基化酶催化去甲基化作用。可以发生在20个常见氨基酸残基中的至少9个(Met, Cys, Lys, Arg, His, Gln, Asn, Glu and Asp)氨基酸中,而最常见甲基化/去甲基化主要发生在赖氨酸(Lys)和精氨酸(Arg)侧链上,几乎参与生物所有的生命活动过程,如调节细胞功能,如转录、细胞分裂和细胞分化。甲基化修饰能够发生在不同的氨基酸位点,或是在同一个氨基酸位点产生不止一个甲基化修饰。
研究发现,常见甲基化/去甲基化作用的氨基酸主要是赖氨酸(Lys)和精氨酸(Arg)研究表明,组蛋白赖氨酸甲基化修饰执行着多种生物学功能,如干细胞的维持和分化、X染色体失活、转录调节和DNA损伤反应等,主要是影响染色质浓缩,抑制基因表达。组蛋白精氨酸甲基化在基因转录调控中发挥着重要作用,并能影响细胞的多种生理过程,包括DNA修复、信号转导、细胞发育及癌症发生等因此专肽生物特地开发甲基化修饰多肽技术,为科学家在蛋白质翻译后修饰(PTMS)的研究中提供帮助。
甲基化修饰(Me1,Me2,Me3)
采用高品质的Fmoc-Lys(Me,Boc)-OH、 Fmoc-Lys(Me2)-OH、Fmoc-Lys(Me3)-OH.HCL、Fmoc-Arg(Me,Pbf)-OH 、Fmoc-Arg(me)2-OH.HCl(asymmetrical) 、Fmoc-Arg(me)2-OH.HCl(symmetrical) 等原料,采用Fmoc固相合成工艺合成,得到Lys甲基化,Arg甲基化标记的多肽,使用HPLC 对产物进行纯化。最终产品提供相应的质谱图,纯度分析的HPLC 色谱图。

多肽H2N-Arg-Pro-Lys-Pro-Gln-Gln-Phe-(NMe)Phe-Sar-Leu-Met-NH2的合成步骤:
1、合成MBHA树脂:取若干克的MBHA树脂(如初始取代度为0.5mmol/g)和1倍树脂摩尔量的Fmoc-Linker-OH加入到反应器中,加入DMF,搅拌使氨基酸完全溶解。再加入树脂2倍量的DIEPA,搅拌混合均匀。再加入树脂0.95倍量的HBTU,搅拌混合均匀。反应3-4小时后,用DMF洗涤3次。用2倍树脂体积的10%乙酸酐/DMF 进行封端30分钟。然后再用DMF洗涤3次,甲醇洗涤2次,DCM洗涤2次,再用甲醇洗涤2次。真空干燥12小时以上,得到干燥的树脂{Fmoc-Linker-MHBA Resin},测定取代度。这里测得取代度为 0.3mmol/g。结构如下图:

2、脱Fmoc:取1.32g的上述树脂,用DCM或DMF溶胀20分钟。用DMF洗涤2遍。加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Linker-MBHA Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:

3、缩合:取1.19mmol Fmoc-Met-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入2.38mmol DIPEA,1.13mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Met-Linker-MBHA Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:

4、依次循环步骤二、步骤三,依次得到
H2N-Met-Linker-MBHA Resin
Fmoc-Leu-Met-Linker-MBHA Resin
H2N-Leu-Met-Linker-MBHA Resin
Fmoc-Sar-Leu-Met-Linker-MBHA Resin
H2N-Sar-Leu-Met-Linker-MBHA Resin
Fmoc-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
H2N-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
Fmoc-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
H2N-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
Fmoc-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
H2N-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
Fmoc-Gln(Trt)-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
H2N-Gln(Trt)-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
Fmoc-Pro-Gln(Trt)-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
H2N-Pro-Gln(Trt)-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
Fmoc-Lys(Boc)-Pro-Gln(Trt)-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
H2N-Lys(Boc)-Pro-Gln(Trt)-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
Fmoc-Pro-Lys(Boc)-Pro-Gln(Trt)-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
H2N-Pro-Lys(Boc)-Pro-Gln(Trt)-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
Fmoc-Arg(Pbf)-Pro-Lys(Boc)-Pro-Gln(Trt)-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Arg(Pbf)-Pro-Lys(Boc)-Pro-Gln(Trt)-Gln(Trt)-Phe-(NMe)Phe-Sar-Leu-Met-Linker-MBHA Resin,结构如下:

5、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品H2N-Arg-Pro-Lys-Pro-Gln-Gln-Phe-(NMe)Phe-Sar-Leu-Met-NH2。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽等。
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





