400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
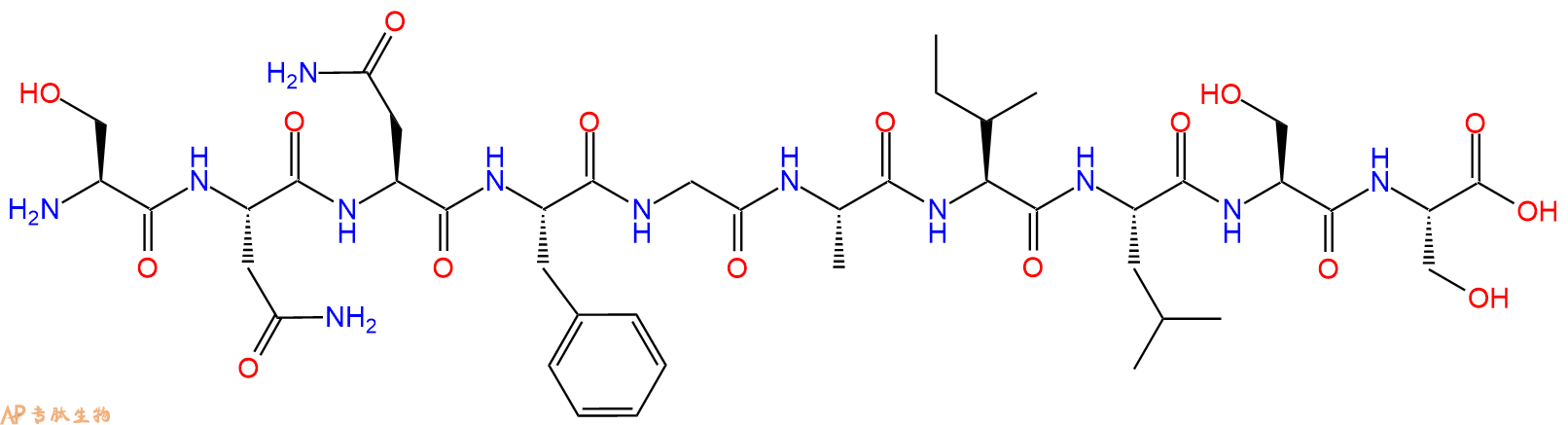
Amylin (20-29) (or hIAPP 20-29) forms fibrils that are ultrastructurally identical to amyloid fibrils seen in pancreatic islets. The region SNNFGAILSS appears to be the most important amyloidogenic sequence of hIAPP (human islet amyloid polypeptide).
编号:192725
CAS号:118068-30-7
单字母:H2N-SNNFGAILSS-OH
Amylin (20-29) (or hIAPP 20-29) forms fibrils that are ultrastructurally identical to amyloid fibrils seen in pancreatic islets. The region SNNFGAILSS appears to be the most important amyloidogenic sequence of hIAPP (human islet amyloid polypeptide).
胰淀素(Amylin)的定义
胰淀素或胰岛淀粉样多肽(IAPP)是一种由胰腺β细胞分泌的37个氨基酸组成的肽,是2型糖尿病患者淀粉样蛋白沉积物的主要成分。胰淀素可被称为胰岛素的“孪生兄弟”,因为它在血浆葡萄糖升高时与胰岛素一起组成性表达。
Amylin or Islet amyloid polypeptide (IAPP), a 37-amino acid peptide is secreted by beta-islet cells of the pancreas and a major component of the amyloid deposits in persons with type 2 diabetes mellitus. Amylin may be referred to as insulin’s “fraternal twin” as it is constitutively expressed with insulin in response to elevations of plasma glucose.
胰淀素(Amylin)的发现
胰岛淀粉样蛋白沉积是糖尿病的主要病理特征,这一知识已为人所知一个世纪。但是,淀粉样蛋白沉积的主要成分胰淀素的发现,是由两个独立的研究小组在1987年完成的【1】【2】。
The knowledge of occurrence of amyloid deposits in islets of Langerhans, major pathologic feature of diabetics has been known for a century. But, the discovery of amylin as a major component of amyloid deposits was by two independent groups in 1987【1】【2】.
胰淀素(Amylin)的结构特征
人胰淀素具有氨基酸序列KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY,其中半胱氨酸残基2和7之间存在二硫键。这些特征、酰胺化的C末端和二硫键对于胰淀素的完全生物活性是必需的【3】。胰淀素的氨基酸序列与降钙素基因相关神经肽CGRP-2和CGRP-1的氨基酸序列分别有46%和43%的相似性。胰淀素的(20-29)片段对胰岛淀粉样蛋白的发病机制至关重要【4】。
The human amylin has an amino acid sequence KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY, with a disulfide bridge between cysteine residues 2 and 7. The features, amidated C-terminus and the disulfide bridge are necessary for the full biological activity of amylin【3】. Amylin amino acid sequence is 46% and 43% identical to those of the calcitonin gene-related neuropeptides CGRP-2 and CGRP-1. The (20-29) fragment of amylin is critical to the pathogenesis of islet amyloid【4】.
胰淀素(Amylin)的作用机制
胰淀素由胰岛β细胞合成,在高尔基体中包装,并在分泌颗粒内分泌。它在肾皮质中的肾小球旁体区域有结合位点,并能激活肾素-血管紧张素-醛固酮系统。此外,它还通过抑制心钠素(ANP)的分泌来作用于循环系统【5】。
Amylin is synthesized, packaged within the golgi apparatus and secreted within the secretory granule by the islet beta cell. They have binding sites within the renal cortex in the area of the juxtaglomerular apparatus and it activates the rennin angiotensin aldosterone system. It also acts upon the circulatory system by inhibiting the secretion of the atrial natriuretic peptide (ANP)【5】.
胰淀素(Amylin)的功能
胰淀素抑制胃排空,在控制和延缓餐后葡萄糖释放速率方面起着重要作用。它抑制餐后肝脏释放和葡萄糖生成。研究还表明,胰淀素能抑制胰高血糖素分泌和生长抑素。胰淀素通过扩张血管的非横纹肌来引起血管舒张。此外,胰淀素还能增加口渴感,这表明它在中枢神经系统内也有作用【6】。
Amylin inhibits gastric emptying and is important in controlling and delaying the rate of meal derived glucose. It inhibits hepatic release and production of glucose in the postprandial period. They also have been shown to inhibit glucagon secretion and somatostatin. Amylin causes vasodilatation by dilating the non-striated muscles of the blood vessels. It is also known to increase thirst level which indicates it has an action within the central nervous system【6】.
胰淀素(Amylin)的相关文献
1. Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB (1987). Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci., 84(23):8628-32.
2. Westermark P, Wernstedt C, O'Brien TD, Hayden DW, Johnson KH (1987). Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am J Pathol, 127(3):414-417.
3. Roberts AN, Leighton B, Todd JA, Cockburn D, Schofield PN, Sutton R, Holt S, Boyd Y, Day AJ, Foot EA, et al,(1989). Molecular and functional characterization of amylin, a peptide associated with type 2 diabetes mellitus. Proc Natl Acad Sci, 86(24):9662-9666.
4. Guidobono F, Pagani F, Ticozzi C, Sibilia V, Pecile A, Netti C (1997). Protection by amylin of gastric erosions induced by indomethacin or ethanol in rats. Br J Pharmacol, 120(4):581-586.
5. Piao FL, Cao C, Han JH, Kim SZ, Cho KW, Kim SH (2004). Amylin-induced suppression of ANP secretion through receptors for CGRP1 and salmon calcitonin. Regul Pept, 117: 59-166.
6. Hayden MR, Tyagi SC (2002). Islet redox stress: the manifold toxicities of insulin resistance, metabolic syndrome and amylin derived islet amyloid in type 2 diabetes mellitus. Journal of the Pancrease, 3(4): 86-108.
| DOI | 名称 | |
|---|---|---|
| 10.1016/S0006-3495(03)70068-X | Micelle formation by a fragment of human islet amyloid polypeptide | 下载 |
| 10.1177/030098589303000401 | Islet amyloid polypeptide: a review of its biology and potential roles in the pathogenesis of diabetes mellitus | 下载 |
多肽H2N-Ser-Asn-Asn-Phe-Gly-Ala-Ile-Leu-Ser-Ser-COOH的合成步骤:
1、合成CTC树脂:称取0.56g CTC Resin(如初始取代度约为0.84mmol/g)和0.56mmol Fmoc-Ser(tBu)-OH于反应器中,加入适量DCM溶解氨基酸(需要注意,此时CTC树脂体积会增大好几倍,避免DCM溶液过少),再加入1.41mmol DIPEA(Mw:129.1,d:0.740g/ml),反应2-3小时后,可不抽滤溶液,直接加入1ml的HPLC级甲醇,封端半小时。依次用DMF洗涤2次,甲醇洗涤1次,DCM洗涤一次,甲醇洗涤一次,DCM洗涤一次,DMF洗涤2次(这里使用甲醇和DCM交替洗涤,是为了更好地去除其他溶质,有利于后续反应)。得到 Fmoc-Ser(tBu)-CTC Resin。结构图如下:

2、脱Fmoc:加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Ser(tBu)-CTC Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:

3、缩合:取1.41mmol Fmoc-Ser(tBu)-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入2.82mmol DIPEA,1.34mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Ser(tBu)-Ser(tBu)-CTC Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:
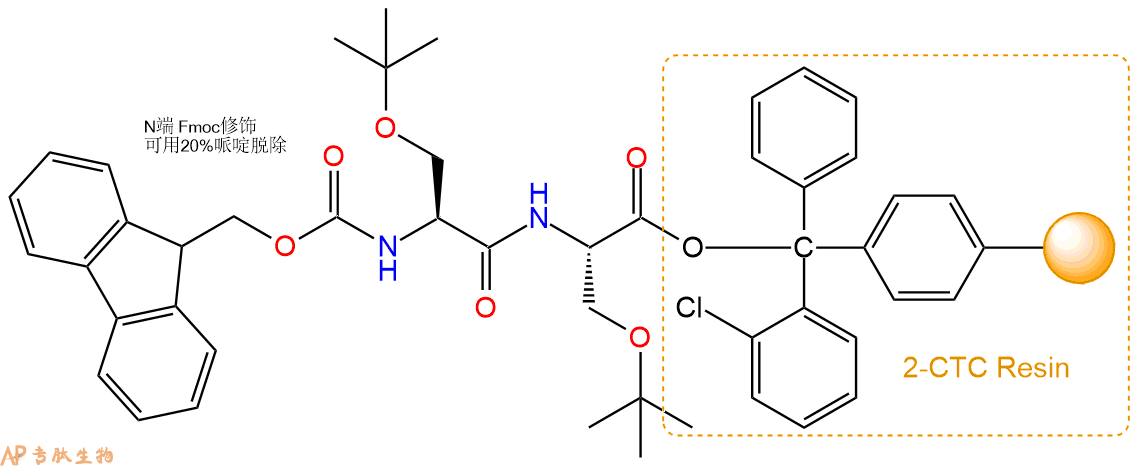
4、依次循环步骤二、步骤三,依次得到
H2N-Ser(tBu)-Ser(tBu)-CTC Resin
Fmoc-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
H2N-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
Fmoc-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
H2N-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
Fmoc-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
H2N-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
Fmoc-Gly-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
H2N-Gly-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
Fmoc-Phe-Gly-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
H2N-Phe-Gly-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
Fmoc-Asn(Trt)-Phe-Gly-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
H2N-Asn(Trt)-Phe-Gly-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
Fmoc-Asn(Trt)-Asn(Trt)-Phe-Gly-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
H2N-Asn(Trt)-Asn(Trt)-Phe-Gly-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
Fmoc-Ser(tBu)-Asn(Trt)-Asn(Trt)-Phe-Gly-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Ser(tBu)-Asn(Trt)-Asn(Trt)-Phe-Gly-Ala-Ile-Leu-Ser(tBu)-Ser(tBu)-CTC Resin,结构如下:

5、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品H2N-Ser-Asn-Asn-Phe-Gly-Ala-Ile-Leu-Ser-Ser-COOH。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%、TFA:H2O=97.5%:2.5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





