
CAS 编号(盐酸戈那瑞林)51952-41-1。
编号:122848
CAS号:35263-73-1
单字母:Pyr-HWSYGLRPG-OH
| 编号: | 122848 |
| 中文名称: | Luteinizing Hormone - Releasing Hormone (LH - RH) free acid human |
| 英文名: | Luteinizing Hormone - Releasing Hormone (LH - RH) free acid human |
| CAS号: | 35263-73-1 |
| 单字母: | Pyr-HWSYGLRPG-OH |
| 三字母: | Pyr 焦谷氨酸 -His组氨酸 -Trp色氨酸 -Ser丝氨酸 -Tyr酪氨酸 -Gly甘氨酸 -Leu亮氨酸 -Arg精氨酸 -Pro脯氨酸 -Gly甘氨酸 -OHC端羧基 |
| 氨基酸个数: | 9 |
| 分子式: | C55H74N16O14 |
| 平均分子量: | 1183.27 |
| 精确分子量: | 1182.56 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | 2.24 |
| 酸性基团个数: | 0.1 |
| 碱性基团个数: | 疏水 |
| 平均亲水性: | -0.96666666666667 |
| 疏水性值: | -0.99 |
| 外观与性状: | 白色粉末状固体 |
| 消光系数: | 6990 |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 促黄体生成激素释放激素(LHRH) |
CAS Number (gonadorelin hydrochloride) 51952-41-1.
Definition
Luteinizing hormone-releasing hormone (LHRH), also known as Gonadotropin-Releasing Hormone (GnRH) or Luteinizing Hormone-Releasing Factor (LRF) is a hypothalamic neuropeptide which acts on the pituitary to stimulate the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
Discovery
LHRH was first characterized by the groups of two Nobel Laureates Guillemin and Schally in 1977. The hypophysiotropic form of the peptide is part of a larger family of decapeptides and was the first to be discovered and characterized in mammals. The discovery of multiple LHRHs in mammalian and non-mammalian species across a wide evolutionary taxa [over 20 LHRHs identified to date] has resulted in a nomenclature based on the species each of the LHRHs was first discovered 1.
Structural Characteristics
LHRH is translated from the mRNA as a pro-hormone, which is subsequently converted to the mature decapeptide (pGlu-His- Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2) in secretory vesicles prior to its release 2. Subsequent to its secretion, LHRH may be further cleaved by soluble peptidases for the purposes of degrading, converting, or transforming 3.
Mode of Action
The effects of LHRH are mediated by high-affinity G protein-coupled LHRH-receptor (LHRH-R) on pituitary gonadotropes. In humans and most vertebrates, there are two forms of LHRHs, LHRH-I and LHRH-II that exist in the brain and in peripheral tissues. Furthermore, the processed peptide of LHRH-I, LHRH-(1–5), appears also to have biological activities that are in contrast to its parent activities. Enormous interest has been focused on LHRH-I and LHRH-II and their cognate receptors as targets for designing therapies to treat cancers of the reproductive system. LHRH-I is processed by a zinc metalloendopeptidase EC 3.4.24.15 (EP24.15) that cleaves the hormone at the fifth and sixth bond of the decapeptide (Tyr5-Gly6) to form LHRH-(1–5). Autoregulation of LHRH gene expression can also be mediated by its processed peptide, LHRH-(1–5). LHRH-(1–5) may act through one of the alternative LHRH receptors, such as the LHRH-RII. This is plausible since LHRH-(1–5) share the first 4 amino acids with at least 9 LHRH-I forms. LHRH-(1–5) have contrasting effects from its parent peptide, LHRH-I, could help explain the lack of correlation between the action of LHRH-I analogs that behave as antagonists at the pituitary level but result in agonist-like anti-proliferative effects in many cancers 4, ,5.
Functions
As growth modulatory factor: LHRH may act as a growth modulatory factor in tumors of the reproductive system. LHRH and LHRH receptors (LHRH-Rs) are expressed in human melanoma cells and LHRH-Rs are found in greater than 50% of human breast cancers 6.
Action on ovary: LHRH is released in pulses and triggers the production of gonadotropins, which stimulate the growth and release of eggs by the ovary. This fact has been best illustrated by experiments in which the actions of the decapeptide have been blocked by immunoneutralization or receptor antagonist treatment, which invariably leads to cessation or reduction of gonadotropin secretion, disruption of gonadal function, and infertility. Sterility in mutant, non-LHRH-producing mice and human infertility associated with LHRH insufficiency also provide a clear demonstration of the reproductive consequences of inappropriate or deficient LHRH neurosecretion 6. In the rat, administration of an LHRH antagonist during proestrus results in a rapid and complete inhibition of ovulation, demonstrating the importance of this neuropeptide in reproductive function.
Hypothalamic anovulation: In women, a disorder called hypothalamic anovulation occurs when the hypothalamus does not produce LHRH, which in turn results in a lack of egg production and release by the ovaries. Similarly, if the LHRH secretion pattern is altered by prolonged stressful situations, a female will show symptoms of dysmenorrhea or amenorrhea, while a male will exhibit alteration of steroidogenesis as well as spermatogenesis.
Reproductive maturation: Regulation of the synthesis and secretion of hypothalamic LHRH neurons plays a fundamental role in the process of reproductive maturation in both mammalian genders. Indeed, an increase in pulsatile LHRH release is the critical factor for the onset of puberty; hypothalamic LHRH content shows a steady increase during the first three months of life in male rats, and an increase in pulsatile LHRH release occurs at the onset of puberty in female rhesus monkeys.
References
1. Walters K, Wegorzewska IN, Chin YP, Parikh MG, Wu TJ (2008). Luteinizing Hormone-Releasing Hormone I (LHRH-I) and Its Metabolite in Peripheral Tissues. Experimental Biology and Medicine, 233 (2):123-130.
2. Wetsel WC, Mellon PL, Weiner RI, Negro-Vilar A (1991). Metabolism of pro-luteinizing hormone-releasing hormone in immortalized hypothalamic neurons. Endocrinology, 129:1584-1595.
3. Wu TJ, Mani SK, Glucksman MJ, Roberts JL (2005). Stimulation of luteinizing hormone-releasing hormone (LHRH) gene expression in GT1–7 cells by its metabolite, LHRH-(1–5). Endocrinology, 146:280–286.
4. Ramakrishnappa N, Rajamahendran R, Lin Y-M, Leung PCK (2005). GnRH in non-hypothalamic reproductive tissues. Anim Reprod Sci., 88:95-113.
5. Swanson TA, Kim SI, Myers M, Pabon A, Philibert KD, Wang M, Glucksman MJ. The role of neuropeptide processing enzymes in endocrine (prostate) cancer: EC 3.4.24.15 (EP24.15). Protein Pept Lett., 11:471-478.
6. Bajo AM, Schally AV, Halmos G, Nagy A (2003). Targeted Doxorubicin-containing Luteinizing Hormone-releasing Hormone Analogue AN-152 Inhibits the Growth of Doxorubicin-resistant MX-1 Human Breast Cancers. Clinical Cancer Research, 9: 3742-3748.
| DOI | 名称 | |
|---|---|---|
| 10.1111/j.1399-3011.1998.tb01475.x | Interaction of gonadotropin-releasing hormone and its agonist analogs with Ca2+ in a nonpolar milieu. Correlation with biopotencies | 下载 |
多肽Pyr-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-COOH的合成步骤:
1、合成CTC树脂:称取1.12g CTC Resin(如初始取代度约为0.87mmol/g)和1.17mmol Fmoc-Gly-OH于反应器中,加入适量DCM溶解氨基酸(需要注意,此时CTC树脂体积会增大好几倍,避免DCM溶液过少),再加入2.92mmol DIPEA(Mw:129.1,d:0.740g/ml),反应2-3小时后,可不抽滤溶液,直接加入1ml的HPLC级甲醇,封端半小时。依次用DMF洗涤2次,甲醇洗涤1次,DCM洗涤一次,甲醇洗涤一次,DCM洗涤一次,DMF洗涤2次(这里使用甲醇和DCM交替洗涤,是为了更好地去除其他溶质,有利于后续反应)。得到 Fmoc-Gly-CTC Resin。结构图如下:

2、脱Fmoc:加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Gly-CTC Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:

3、缩合:取2.92mmol Fmoc-Pro-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入5.85mmol DIPEA,2.78mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Pro-Gly-CTC Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:

4、依次循环步骤二、步骤三,依次得到
H2N-Pro-Gly-CTC Resin
Fmoc-Arg(Pbf)-Pro-Gly-CTC Resin
H2N-Arg(Pbf)-Pro-Gly-CTC Resin
Fmoc-Leu-Arg(Pbf)-Pro-Gly-CTC Resin
H2N-Leu-Arg(Pbf)-Pro-Gly-CTC Resin
Fmoc-Gly-Leu-Arg(Pbf)-Pro-Gly-CTC Resin
H2N-Gly-Leu-Arg(Pbf)-Pro-Gly-CTC Resin
Fmoc-Tyr(tBu)-Gly-Leu-Arg(Pbf)-Pro-Gly-CTC Resin
H2N-Tyr(tBu)-Gly-Leu-Arg(Pbf)-Pro-Gly-CTC Resin
Fmoc-Ser(tBu)-Tyr(tBu)-Gly-Leu-Arg(Pbf)-Pro-Gly-CTC Resin
H2N-Ser(tBu)-Tyr(tBu)-Gly-Leu-Arg(Pbf)-Pro-Gly-CTC Resin
Fmoc-Trp(Boc)-Ser(tBu)-Tyr(tBu)-Gly-Leu-Arg(Pbf)-Pro-Gly-CTC Resin
H2N-Trp(Boc)-Ser(tBu)-Tyr(tBu)-Gly-Leu-Arg(Pbf)-Pro-Gly-CTC Resin
Fmoc-His(Trt)-Trp(Boc)-Ser(tBu)-Tyr(tBu)-Gly-Leu-Arg(Pbf)-Pro-Gly-CTC Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-His(Trt)-Trp(Boc)-Ser(tBu)-Tyr(tBu)-Gly-Leu-Arg(Pbf)-Pro-Gly-CTC Resin,结构如下:
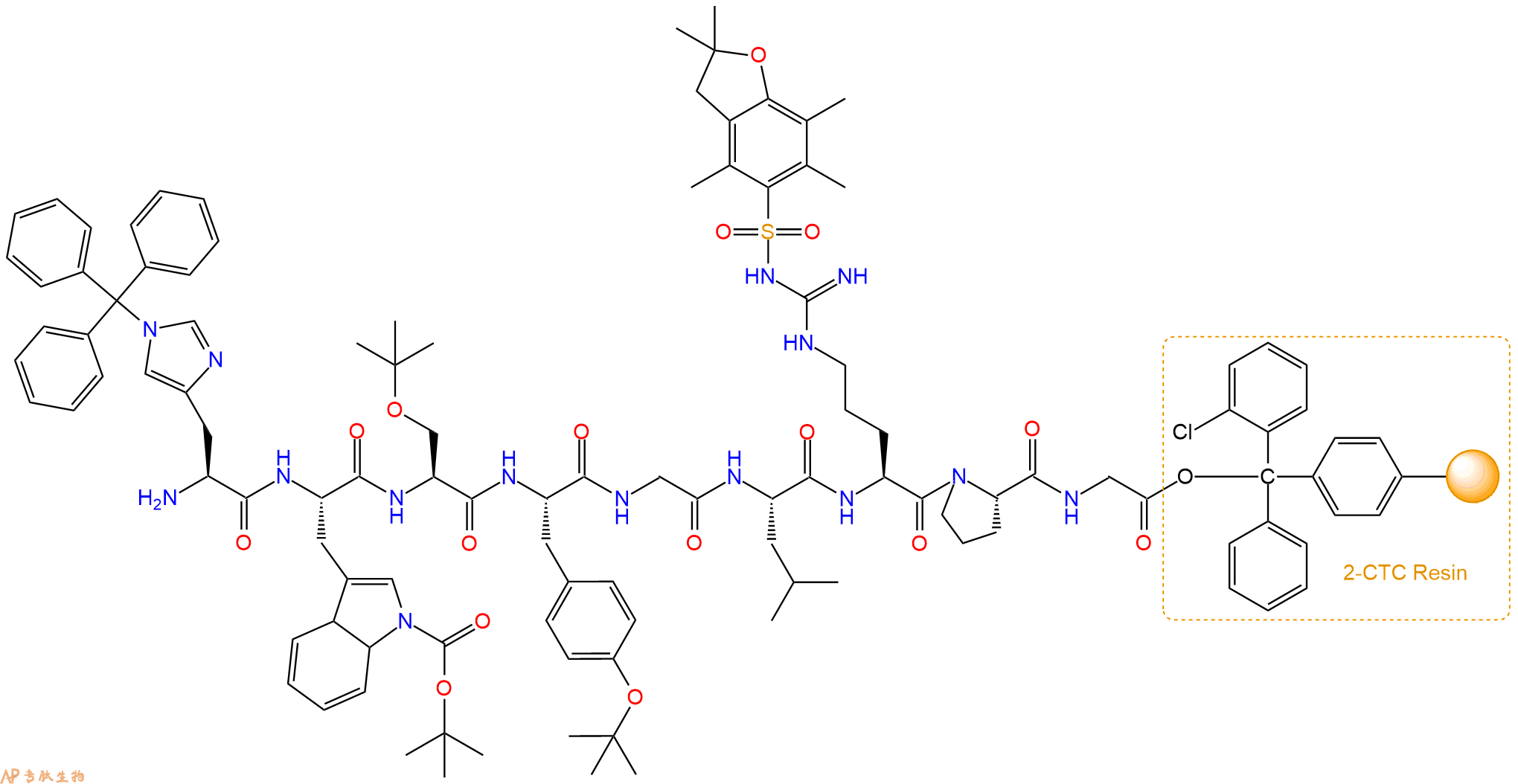
5、焦谷氨酸反应连接:在上述树脂中,加入适当DMF后,再加入2.92mmol 焦谷氨酸到树脂中,再加入5.85mmol DIPEA、2.78mmol HBTU,鼓氮气反应30分钟。用2倍树脂体积的DMF 洗涤3次树脂(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。 得到Pyr-His(Trt)-Trp(Boc)-Ser(tBu)-Tyr(tBu)-Gly-Leu-Arg(Pbf)-Pro-Gly-CTCResin。 结构如下:
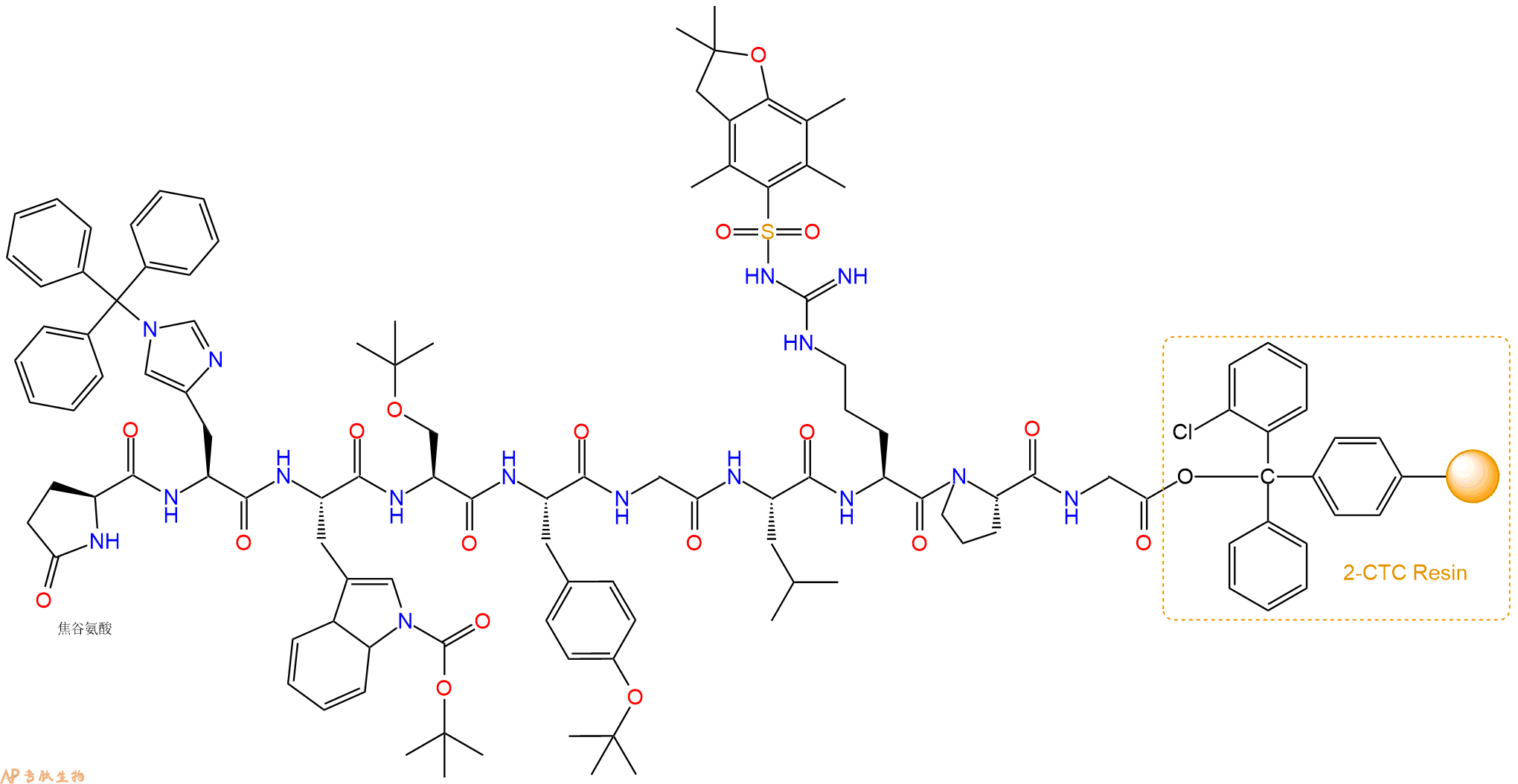
6、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品Pyr-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-COOH。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





