400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研

PR-39是一种富含脯氨酸和精氨酸的天然抗菌肽,是一种非竞争性,可逆和变构的蛋白酶体 (proteasome) 抑制剂。
编号:200653
CAS号:
单字母:H2N-RRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPPRFPPRFP-CONH2
PR-39 TFA 是富含脯氨酸和精氨酸的天然抗菌肽,是一种非竞争性,可逆和变构的蛋白酶体 (proteasome) 抑制剂。PR-39 TFA 可逆地结合到蛋白酶体的 α7 亚基上,并通过泛素-蛋白酶体途径阻断 NF-κB 抑制剂 IκBα 的降解。PR-39 TFA 刺激小鼠的血管生成,抑制炎症反应并显着减小心肌梗死面积。
PR-39 TFA, a natural proline- and arginine-rich antibacterial peptide, is a noncompetitive, reversible and allosteric proteasome inhibitor. PR-39 TFAreversibly binds to the α7 subunit of the proteasome and blocks degradation of NF-κB inhibitor IκBα by the ubiquitin-proteasome pathway. PR-39 TFA stimulates angiogenesis, inhibits inflammatory responses and significant reduces myocardial infarct size in mice[1][2].
抗菌肽介绍一
AMPs是由相对较小的分子组成的异质基团,通常含有不到100个氨基酸。 它们最初是在20世纪60年代由Zeya和Spitznagel 在多形核白细胞溶酶体中描述的。 迄今为止,已在数据库(如数据库)中 确定和登记了2600多个AMP。 它们是由几乎所有的生物群产生的,包括细菌、真菌、植物和动物。 许多脊椎动物AMPs是由上皮表面分泌的,如 哺乳动物的气管、舌、肠粘膜或两栖动物的皮肤。 有些在中性粒细胞、单核 细胞和巨噬细胞中表达。 AMPs参与动物和植物的免疫防御系统。 构成表达或诱导它们在抵御微生物入侵者 的第一道防线中起着关键作用。
结构/分类 AMPs可以根据其氨基酸组成和结构进行分类。 可以区分两大类AMP。
第一类由线性分子组成,它们要么倾向于采用α螺旋结构,要么富含精氨酸、甘氨 酸、组氨酸、脯氨酸和色氨酸等某些氨 基酸。
第二类由含半胱氨酸的肽组成, 可分为单一或多个二硫结构。 在许多情 况下,抗菌活性需要存在二硫桥。 大多数AMPs是阳离子肽,但也有阴离子肽,如真皮素,一种富含天冬氨酸 的人肽和两栖动物的最大蛋白H5皮肤。 其他非阳离子AMPs包括神经肽前体分子的片段,如原啡肽A, 芳香二肽主要从二翅目幼虫中分离出来,或从节肢动物或茴香物种的氧结合 蛋白中提取的肽。
专肽生物可定制合成各类序列的抗菌肽,可标记FITC/FAM/TAMRA等常见荧光素。
Definition
Antimicrobial peptides (AMPs) are as widespread as bacterial inactivator molecules in the innate immune systems of insects, fungi, plants, and mammals. These peptides are also known as host defense peptides (HDPs) as they have other immuno-modulatory functions besides the direct antimicrobial actions and are even capable of killing cancerous cells 1,2.
Classification
Three broad categories of HDPs have been identified: 1) the linear peptides with helical structures, 2) the cysteine stabilized peptides with beta-sheet, and 3) a group of linear peptides rich in proline and arginine that primarily have been identified in non-mammalian species.
Structural characteristics
In mammals, cathelicidins and defensins are the two principal AMP families. Cathelicidins are peptides with a conserved proregion and a variable C-terminal antimicrobial domain. Defensins are the best-characterized AMPs, they have six invariant cysteines, forming three intramolecular cystine-disulfide bonds.
Mode of action
The mode of action of AMPs elucidated to date include inhibition of cell wall formation, formation of pores in the cell membrane resulting in the disruption of membrane potential with eventual lysis of the cell. These peptides also inhibit nuclease activity of both RNase and DNase.
Functions
They have a broad ability to kill microbes. AMPs form an important means of host defense in eukaryotes. Large AMPs (>100 amino acids), are often lytic, nutrient-binding proteins or specifically target microbial macromolecules. Small AMPs act by disrupting the structure of microbial cell membranes. It plays an active role in wound repair and regulation of the adaptive immune system. They have multiple roles as mediators of inflammation with impact on epithelial and inflammatory cells, influencing diverse processes such as cell proliferation, wound healing, cytokine release, chemotaxis and immune induction 3.
References
1. Gottlieb CT, Thomsen LE, Ingmer H, Mygind PH, Kristensen HH, Gram L(2008). Antimicrobial peptides effectively kill a broad spectrum of Listeria monocytogenes and Staphylococcus aureus strains independently of origin, sub-type, or virulence factor expression. BMC Microbiol., 8:205.
2. Yeaman MR and Yount NY (2003). Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmocological Reviews, 55(1).
3. Hanna Galkowska H and Olszewski WL (2003). Antimicrobial peptides – their role in immunity and therapeutic potential. Centr Eur J Immunol., 28 (3):138–141.
抗菌肽介绍二
Ribosomally synthesized antimicrobial peptides (AMPs) constitute a structurally diverse group of molecules found virtually in all organisms. Most antimicrobial peptides contain less than 100 amino acid residues, have a net positive charge, and are membrane active. They are major players in the innate immune defense but can also have roles in processes as chemokine induction, chemotaxis, inflammation, and wound healing. In addition to their antimicrobial effects, many of them show antiviral and antineoplastic activities.
INTRODUCTION
AMPs are a heterogeneous group of relatively small molecules usually containing less than a hundred amino acids. They were first described in the 1960’s by Zeya and Spitznagel in polymorphonuclear leukocyte lysosomes.
To date, more than 2600 AMPs have been identified and registered in databases. They are produced by nearly all groups of organisms, including bacteria, fungi, plants, and animals. Many vertebrate AMPs are secreted by epithelial surfaces such as the tracheal, lingual, or intestinal mucosa of mammals or the skin of amphibia. Some are expressed in neutrophils, monocytes, and macrophages.
AMPs are involved in both animal and plant immune defense systems. Constitutively expressed or induced they play a key role in the first line of defense against microbial intruders.
STRUCTURE/CLASSIFICATION
AMPs can be classified on the basis of their amino acid composition and structure. Two major groups of AMPs can be distinguished. The first group consists of linear molecules which either tend to adopt α-helical structure or are enriched in certain amino acids such as arginine, glycine, histidine, proline, and tryptophan. The second group consists of cysteine-containing peptides which can be divided into single or multiple disulfide structures. In many cases, the presence of disulfide bridges is required for antimicrobial activity.
Most AMPs are cationic peptides, but there are also anionic peptides such as dermcidin, an aspartic acid-rich peptide from human and maximin H5 from amphibian skin. Other non-cationic AMPs include fragments from neuropeptide precursor molecules such as proenkephalin A, aromatic dipeptides primarily isolated from dipteran larvae, or peptides derived from oxygen-binding proteins from arthropod or annelid species.
MODE OF ACTION
Most AMPs act by provoking an increase in plasma membrane permeability. They preferentially target microbial versus mammalian cells. Selectivity is influenced by several factors such as differences in membrane composition: membranes of many bacterial pathogens contain negatively charged lipid moieties such as phosphatidylglycerol (PG), cardiolipin, and phosphatidylserine (PS), whereas mammalian membranes, commonly enriched in phosphatidylethanolamine (PE), phosphatidylcholine (PC) and sphingomyelin, are generally neutral in net charge.
The presence of sterols such as cholesterol and ergesterol within the membrane may be a further means by which AMPs can distinguish between mammalian or fungal cells and prokaryotes. A first step in the mechanism of membrane permeabilization is the electrostatic interaction between the positively charged AMP with the negatively charged membrane surface of the microorganism. Subsequent disruption of the membrane by creation of pores within the microbial membrane ultimately results in cell death of the organism due to leakage of ions, metabolites, cessation of membrane-coupled respiration, and biosynthesis.
Several models for pore formation such as the Barrel-Stave, the Toroidal or Wormhole Model, and the Carpet Model have been proposed (Fig. 1).
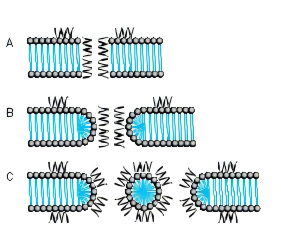
FIG. 1. MODE OF ACTION A BARREL-STAVE MODEL B TOROIDAL PORE OR WORMHOLE MODEL C CARPET MODEL
THE BARREL-STAVE MODEL
The Barrel-Stave model describes a mechanism in which AMPs form a barrellike pore within the bacterial membrane with the individual AMPs or AMP complexes being the staves. Arranged in this manner, the hydrophobic regions of the AMPs point outwards towards the acyl chains of the membrane whereas the hydrophilic areas form the pore.
THE TOROIDAL PORE OR WORMHOLE MODEL
The pores described by this model differ from those of the Barrel-Stave model. Primarily, the outer and inner leaflet of the membrane are not intercalated in the transmembrane channel.
THE CARPET MODEL
A different mechanism is proposed in the Carpet model where AMPs first cover the outer surface of the membrane and then disrupt the membrane like detergents by forming micelle-like units. Certain AMPs penetrate the bacterial membrane without channel formation. They act on intracellular targets by e.g. inhibiting nucleic acid and/or protein synthesis.
RESISTANCE
Resistance to AMPs can either be constitutive or inducible. Inherited resistance mechanisms include altered surface charge, active efflux, production of peptidases or trapping proteins, and modification of host cellular processes. For instance, Staphylococcus aureus manages to reduce the overall cell surface charge by esterification of the cell wall component teichoic acid with D-alanine and thereby increases its resistance against human AMPs. Another example for changing the surface net charge is the production of cationic lysine-substituted phosphatidylglycerol (L-PG) found in certain Staphylococcus aureus strains. In Gram-negative bacteria, addition of 4-aminoarabinose (Ara4N) to the phosphate group of the lipid A backbone or increased acylation of lipopolysaccharides (LPS) are important mechanisms of AMP resistance. Exposure to AMPs may also induce stress responses by which microorganisms try to survive. Inducible regulatory mechanisms have been described in a variety of organisms. For instance, the PhoP/PhoQ regulon in Salmonella has been demonstrated to regulate transcriptional activation of surface and secretory proteins, enzymes that modify lipopolysaccharide, lipid and protein constituents of the outer membrane and proteases that likely degrade certain AMPs.
EXAMPLES OF ANTIMICROBIAL PEPTIDES
| Cationic peptides enriched for specific amino acids |
|
|---|---|
| Glycine-containing peptides | Hymenoptaecin from honeybees |
| Glycine- and proline-containing peptides | Coleoptericin from beetles Holotricin from beetles |
| Histidine-containing peptides | Histatins from humans and some higher primates |
| Proline-containing peptides | Abaecin from honeybees |
| Proline- and arginine-containing peptides | Apidaecins from honeybees Bactenicins from cattle Drosocin from Drosophila PR-39 from pigs |
| Proline- and phenylalanine-containing peptides | Prophenin from pigs |
| Tryptophan-containing peptides | Indolicidin from cattle |
| Linear cationic α-helical peptides | |
|---|---|
| Andropin from insects Bombinin from amphibians Buforin II from amphibians CAP18 from rabbits Cepropins from insects Cecropin P1 from the pig intestinal parasitic nematode, Ascaris suum Ceratotoxin from insects Dermaseptin from amphibians LL-37 from human Magainin from amphibians Melittin from insects Pleurocidin from Pseudopleuronectes americanus |
| Anionic and cationic peptides that contain cysteine and form disulfide bonds |
|
|---|---|
| 1 Disulfide bond | Brevinins |
| 2 Disulfide bonds | Protegrins from pigs |
| 3 Disulfide bonds | α-Defensins from human, rabbits and rats β-Defensins from humans, cattle, mice, rats, pigs, goats and poultry θ-Defensin from the rhesus monkey Insect defensins (Defensin-A from Aedes aegypti) |
| 4 Disulfide bonds | Antifungal defensins from plants Drosomycin from Drosophila |
| Anionic peptides | Dermcidin from human skin Maximin H5 from amphibian skin |
| Anionic and cationic peptide fragments derived from precursor proteins |
Antimicrobial domains from bovine α-lactalbumin, human hemoglobin, lysozyme, and ovalbumin Aromatic dipeptides from dipteran larvae Casocidin I from human casein Enkelytin from proenkaphalin A Lactoferricin from lactoferrin |
ADAPTED FROM K.A. BROGDEN, NAT. REV. MICROBIOL. 3, 238-250 (2005)
IMPORTANT FAMILIES OF AMPS
BOMBININS
Bombinins constitute a family of AMPs produced in fire-bellied toads (Bombina species) active against Gram-negative and Gram-positive bacteria and fungi. Bombinins, bombinin-like peptides (BLPs), and Bombinin H molecules are found in the species Bombina bombina, Bombina variegata, and Bombina orientalis, whereas the homologous maximins and maximin H peptides are derived from the giant fire-bellied toad Bombina maxima. Bombinin H peptides contain either 17 or 20 amino acid residues and are more hydrophobic than bombinins, some of them contain D-alloisoleucine at position 2. They exhibit lower antibacterial activity than bombinins but, in contrast to them, they possess haemolytic activity.
CATHELICIDINS
Members of this family are amphipathic, cationic peptides with a broad-spectrum antimicrobial activity. Cathelicidins typically act by disrupting the integrity of bacterial membranes. They are characterized by an evolutionary conserved N-terminal cathelin- like domain of approximately 99-114 amino acid residues linked to a C-terminal antimicrobial domain of 12-100 residues that can be released upon proteolytic processing. Members of this family include linear peptides amongst them a number of proline-rich AMPs that show different types of proline repeat motifs (Bac5, Bac7, PR-39, prophenins) and the tryptophan-rich indolicidin characterized by three regularly spaced proline residues. The protegrins (PG-1 to PG-5) contain two disulfide bridges and an amidated C-terminus. Cathelicidins have been found in every mammalian species examined. In human, LL-37 (Product 4042456) is the only member of the cathelicidin family. The peptide consists of 37 amino acids and contains two leucine residues at the N-terminus. It is proteolytically cleaved from the 18 kDa precursor protein human cathelicidin antimicrobial protein CAP-18. LL-37 is primarily produced by phagocytic leucocytes and epithelial cells, and is involved in various processes such as direct killing of microorganisms, binding and neutralizing LPS, chemotaxis and chemokine induction, regulation of inflammatory responses, and wound healing. Its production is influenced by several factors such as microbial products, host cytokines, vitamin D3, and availability of oxygen. LL-37 orthologues in mouse and rat are CRAMP (mouse) (Product 4056438) and CRAMP (rat), respectively.
CECROPINS
Cecropins were first isolated from the giant silk moth Hyalophora cecropia. They can form amphipathic, α-helical structures and are structurally related to other cecropins as bactericidin, lepidopteran, and sarcotoxin. Cecropin P1 (Product 4039862), found in pig intestine, also belongs to this family. Most cecropins show broad-spectrum antibacterial activity. Cecropin A (Product 4030488) and B (Product 4030477) have also been demonstrated to possess tumoricidal activity against mammalian leukemia, lymphoma, and carcinoma cell lines.
CERATOTOXINS
This family consists of cationic α-helical amphipathic peptides expressed in the female reproductive accessory glands of the Mediterranean fruit fly Ceratitis capitata. The production of the peptides is enhanced by mating. Ceratotoxin A and ceratotoxin B are 29 amino acid peptides differing in two amino acids. Ceratotoxin C and D consist of 32 and 36 amino acids, respectively. The peptides of this family are active against Gram-negative as well as Grampositive bacteria and are supposed to act via the Barrel-Stave model. Ceratotoxin A has been shown to be mainly antibacterial for Gram-negative organisms.
DEFENSINS
Defensins are small cysteine-rich cationic peptides containing three or four disulfide bridges. They have been isolated from molluscs, acari, arachnids, insects, mammals, and plants. They are further divided into families on the basis of the spatial distribution of their cysteine residues. Three families, the α-, β- and θ-defensins, can be distinguished in mammals. α- and β-defensins are characterized by antiparallel β-sheet structures stabilized by three disulfide bonds. The θ-defensins are found in rhesus monkey and some other non-human primates but not in human, chimpanzee and gorilla. They consist of two nine amino acid peptides derived from different precursor proteins joined by head-to-tail cyclization. Invertebrate and plant defensins contain three or four disulfide bridges, respectively. Insect and mammalian defensins are mainly active against bacteria while most plant defensins possess antifungal activity.
DERMASEPTINS
The peptides of the dermaseptin family are closely related and consist of 28-34 amino acids. They were originally isolated from skin extracts of the South American arboreal frog Phyllomedusa sauvagei and contain a conserved tryptophan residue at position 3. Dermaseptins exhibit broad-spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria.
HISTATINS
Histatins are histidine-rich and mostly cationic peptides found in the saliva of humans and some higher primates. They are active against a broad-spectrum of bacteria and fungi. The antifungal activity of the human salivary peptide histatin-5 has been extensively studied and is supposed to be due to inhibition of mitochondrial respiration and the formation of reactive oxygen species. Histatin-5 has also been shown to inhibit both host-derived and bacterial proteolytc enzymes involved in peridontal diseases. Histatin-8, a peptide from human parotid secretion, has been shown to inhibit hemagglutination activity of Porphyromonas gingivalis 381, a Gram-negative bacterium involved in certain forms of periodontal disease. The peptide may function as a binding domain for the hemagglutinins of Porphyromonas gingivalis during agglutination.
MAGAININS
Magainins constitute a family of linear amphipathic cationic AMPs discovered in the skin of Xenopus laevis. The two closely related members of this family, magainin I (Product 4012844) and magainin II (Product 4013706) differ merely in two positions and are 23 amino acids in length. Magainins exhibit broad-spectrum antimicrobial activity against Gram-negative and Gram-positive bacteria, fungi and protozoa and are also cytotoxic for many murine and human cancer cell lines.
CONCLUSIONS
The structures of AMPs represent a unique source for the targeted exploration of new applications in the therapy of microbial and viral infection, cancer, and sepsis. Modern synthetic methods will allow the relatively cheap and accurate production of lead compounds and peptide candidates. The achievements in peptide library generation, analytical methods as mass spectrometry, and screening and formulation technologies may contribute to solve intrinsic problems associated with the use of AMPs as therapeutic agents such as susceptibility to proteases and host toxicity. Bachem has considerable expertise and long-standing experience in peptide synthesis. With our capacity to upscale the production of simple and modified peptides, we are the partner of choice for the pharmaceutical industries.
定义
酶是用于生化反应的非常有效的催化剂。它们通过提供较低活化能的替代反应途径来加快反应速度。酶作用于底物并产生产物。一些物质降低或什至停止酶的催化活性被称为抑制剂。
发现
1965年,Umezawa H分析了微生物产生的酶抑制剂,并分离出了抑制亮肽素和抗痛药的胰蛋白酶和木瓜蛋白酶,乳糜蛋白酶抑制的胰凝乳蛋白酶,胃蛋白酶抑制素抑制胃蛋白酶,泛磷酰胺抑制唾液酸酶,乌藤酮抑制酪氨酸羟化酶,多巴汀抑制多巴胺3-羟硫基嘧啶和多巴胺3-羟色胺酶酪氨酸羟化酶和多巴胺J3-羟化酶。最近,一种替代方法已应用于预测新的抑制剂:合理的药物设计使用酶活性位点的三维结构来预测哪些分子可能是抑制剂1。已经开发了用于识别酶抑制剂的基于计算机的方法,例如分子力学和分子对接。
结构特征
已经确定了许多抑制剂的晶体结构。已经确定了三种与凝血酶复合的高效且选择性的低分子量刚性肽醛醛抑制剂的晶体结构。这三种抑制剂全部在P3位置具有一个新的内酰胺部分,而对胰蛋白酶选择性最高的两种抑制剂在P1位置具有一个与S1特异性位点结合的胍基哌啶基。凝血酶的抑制动力学从慢到快变化,而对于胰蛋白酶,抑制的动力学在所有情况下都快。根据两步机理2中稳定过渡态络合物的缓慢形成来检验动力学。
埃米尔•菲舍尔(Emil Fischer)在1894年提出,酶和底物都具有特定的互补几何形状,彼此恰好契合。这称为“锁和钥匙”模型3。丹尼尔·科什兰(Daniel Koshland)提出了诱导拟合模型,其中底物和酶是相当灵活的结构,当底物与酶4相互作用时,活性位点通过与底物的相互作用不断重塑。
在众多生物活性肽的成熟过程中,需要由其谷氨酰胺(或谷氨酰胺)前体形成N末端焦谷氨酸(pGlu)。游离形式并与底物和三种咪唑衍生抑制剂结合的人QC的结构揭示了类似于两个锌外肽酶的α/β支架,但有多个插入和缺失,特别是在活性位点区域。几种活性位点突变酶的结构分析为针对QC相关疾病5的抑制剂的合理设计提供了结构基础。
作用方式
酶是催化化学反应的蛋白质。酶与底物相互作用并将其转化为产物。抑制剂的结合可以阻止底物进入酶的活性位点和/或阻止酶催化其反应。抑制剂的种类繁多,包括:非特异性,不可逆,可逆-竞争性和非竞争性。可逆抑制剂 以非共价相互作用(例如疏水相互作用,氢键和离子键)与酶结合。非特异性抑制方法包括最终使酶的蛋白质部分变性并因此不可逆的任何物理或化学变化。特定抑制剂 对单一酶发挥作用。大多数毒药通过特异性抑制酶发挥作用。竞争性抑制剂是任何与底物的化学结构和分子几何结构非常相似的化合物。抑制剂可以在活性位点与酶相互作用,但是没有反应发生。非竞争性抑制剂是与酶相互作用但通常不在活性位点相互作用的物质。非竞争性抑制剂的净作用是改变酶的形状,从而改变活性位点,从而使底物不再能与酶相互作用而产生反应。非竞争性抑制剂通常是可逆的。不可逆抑制剂与酶形成牢固的共价键。这些抑制剂可以在活性位点附近或附近起作用。
功能
工业应用中, 酶在商业上被广泛使用,例如在洗涤剂,食品和酿造工业中。蛋白酶用于“生物”洗衣粉中,以加速蛋白质在诸如血液和鸡蛋等污渍中的分解。商业上使用酶的问题包括:它们是水溶性的,这使得它们难以回收,并且一些产物可以抑制酶的活性(反馈抑制)。
药物分子,许多药物分子都是酶抑制剂,药用酶抑制剂通常以其特异性和效力为特征。高度的特异性和效力表明该药物具有较少的副作用和较低的毒性。酶抑制剂在自然界中发现,并且也作为药理学和生物化学的一部分进行设计和生产6。
天然毒物 通常是酶抑制剂,已进化为保护植物或动物免受天敌的侵害。这些天然毒素包括一些已知最剧毒的化合物。
神经气体( 例如二异丙基氟磷酸酯(DFP))通过与丝氨酸的羟基反应生成酯,从而抑制了乙酰胆碱酯酶的活性位点。
参考
1、Scapin G (2006). Structural biology and drug discovery. Curr. Pharm. Des., 12(17):2087–2097.
2、Krishnan R, Zhang E, Hakansson K, Arni RK, Tulinsky A, Lim-Wilby MS, Levy OE, Semple JE, Brunck TK (1998). Highly selective mechanism-based thrombin inhibitors: structures of thrombin and trypsin inhibited with rigid peptidyl aldehydes. Biochemistry, 37 (35):12094-12103.
3、Fischer E (1894). Einfluss der configuration auf die wirkung der enzyme. Ber. Dt. Chem. Ges., 27:2985–2993.
4、Koshland DE (1958). Application of a theory of enzyme specificity to protein synthesis. PNAS., 44 (2):98–104.
5、Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH (2005). Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. PNAS., 102(37):13117-13122.
6、Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (2002). Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins. Curr Med Chem., 9(22):1981-1989.
Definition
Enzymes are very efficient catalysts for biochemical reactions. They speed up reactions by providing an alternative reaction pathway of lower activation energy. Enzyme acts on substrate and gives rise to a product. Some substances reduce or even stop the catalytic activities of enzymes are called inhibitors.
Discovery
In 1965, Umezawa H analysed enzyme inhibitors produced by microorganisms and isolated leupeptin and antipain inhibiting trypsin and papain, chymostatin inhibiting chymotrypsin, pepstatin inhibiting pepsin, panosialin inhibiting sialidases, oudenone inhibiting tyrosine hydroxylase, dopastin inhibiting dopamine 3-hydroxylase, aquayamycin and chrothiomycin inhibiting tyrosine hydroxylase and dopamine J3-hydroxylase . Recently, an alternative approach has been applied to predict new inhibitors: rational drug design uses the three-dimensional structure of an enzyme's active site to predict which molecules might be inhibitors 1. Computer-based methods for identifying inhibitor for an enzyme have been developed, such as molecular mechanics and molecular docking.
Structural Characteristics
The crystal structures of many inhibitors have been determined. The crystal structures of three highly potent and selective low-molecular weight rigid peptidyl aldehyde inhibitors complexed with thrombin have been determined. All the three inhibitors have a novel lactam moiety at the P3 position, while the two with greatest trypsin selectivity have a guanidinopiperidyl group at the P1 position that binds in the S1 specificity site. The kinetics of inhibition vary from slow to fast with thrombin and are fast in all cases with trypsin. The kinetics are examined in terms of the slow formation of a stable transition-state complex in a two-step mechanism 2.
Emil Fischer in 1894 suggested that both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another.This is known as "the lock and key" model 3. Daniel Koshland suggested induced fit model where substrate and enzymes are rather flexible structures, the active site is continually reshaped by interactions with the substrate as the substrate interacts with the enzyme 4.
N-terminal pyroglutamate (pGlu) formation from its glutaminyl (or glutamyl) precursor is required in the maturation of numerous bioactive peptides. The structure of human QC in free form and bound to a substrate and three imidazole-derived inhibitors reveals an alpha/beta scaffold akin to that of two-zinc exopeptidases but with several insertions and deletions, particularly in the active-site region. The structural analyses of several active-site-mutant enzymes provide a structural basis for the rational design of inhibitors against QC-associated disorders 5.
Mode of Action
Enzymes are proteins that catalyze chemical reactions. Enzymes interact with substrate and convert them into products. Inhibitor binding can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. There are a variety of types of inhibitors including: nonspecific, irreversible, reversible - competitive and noncompetitive. Reversible inhibitors bind to enzymes with non-covalent interactions like hydrophobic interactions, hydrogen bonds, and ionic bonds. Non-specific methods of inhibition include any physical or chemical changes which ultimately denature the protein portion of the enzyme and are therefore irreversible. Specific Inhibitors exert their effects upon a single enzyme. Most poisons work by specific inhibition of enzymes. A competitive inhibitor is any compound which closely resembles the chemical structure and molecular geometry of the substrate. The inhibitor may interact with the enzyme at the active site, but no reaction takes place. A noncompetitive inhibitor is a substance that interacts with the enzyme, but usually not at the active site. The net effect of a non competitive inhibitor is to change the shape of the enzyme and thus the active site, so that the substrate can no longer interact with the enzyme to give a reaction. Non competitive inhibitors are usually reversible. Irreversible Inhibitors form strong covalent bonds with an enzyme. These inhibitors may act at, near, or remote from the active site .
Functions
Industrial application, enzymes are widely used commercially, for example in the detergent, food and brewing industries. Protease enzymes are used in 'biological' washing powders to speed up the breakdown of proteins in stains like blood and egg. Problems using enzymes commercially include: they are water soluble which makes them hard to recover and some products can inhibit the enzyme activity (feedback inhibition) .
Drug molecules, many drug molecules are enzyme inhibitors and a medicinal enzyme inhibitor is usually characterized by its specificity and its potency. A high specificity and potency suggests that a drug will have fewer side effects and less toxic. Enzyme inhibitors are found in nature and are also designed and produced as part of pharmacology and biochemistry 6.
Natural poisons are often enzyme inhibitors that have evolved to defend a plant or animal against predators. These natural toxins include some of the most poisonous compounds known.
Nerve gases such as diisopropylfluorophosphate (DFP) inhibit the active site of acetylcholine esterase by reacting with the hydroxyl group of serine to make an ester.
References
Scapin G (2006). Structural biology and drug discovery. Curr. Pharm. Des., 12(17):2087–2097.
Krishnan R, Zhang E, Hakansson K, Arni RK, Tulinsky A, Lim-Wilby MS, Levy OE, Semple JE, Brunck TK (1998). Highly selective mechanism-based thrombin inhibitors: structures of thrombin and trypsin inhibited with rigid peptidyl aldehydes. Biochemistry, 37 (35):12094-12103.
Fischer E (1894). Einfluss der configuration auf die wirkung der enzyme. Ber. Dt. Chem. Ges., 27:2985–2993.
Koshland DE (1958). Application of a theory of enzyme specificity to protein synthesis. PNAS., 44 (2):98–104.
Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH (2005). Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. PNAS., 102(37):13117-13122.
Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (2002). Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins. Curr Med Chem., 9(22):1981-1989.
Maria Gaczynska, et al. Proline- and arginine-rich peptides constitute a novel class of allosteric inhibitors of proteasome activity. Biochemistry. 2003 Jul 29;42(29):8663-70. : https://pubmed.ncbi.nlm.nih.gov/12873125/
Y Gao, et al. Inhibition of ubiquitin-proteasome pathway-mediated I kappa B alpha degradation by a naturally occurring antibacterial peptide. J Clin Invest. 2000 Aug;106(3):439-48. : https://pubmed.ncbi.nlm.nih.gov/10930447/





