400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研

C-反应蛋白的 201-206 片段。C-Reactive Protein 可作为炎症的标记,是心血管风险的标志物,可能促进动脉粥样硬化形成。
编号:146738
CAS号:130348-99-1/2918771-38-5
单字母:H2N-KPQLWP-OH
| 参考文献(References): | R.M.Heuertz et al., J. Immunol., 156, 3412 (1996) |
C-Reactive Protein (CRP) 201-206 是 C-反应蛋白的 201-206 片段。C-Reactive Protein 可作为炎症的标记,是心血管风险的标志物,可能促进动脉粥样硬化形成。
C-Reactive Protein (CRP) 201-206 is the 201-206 fragment of C-Reactive Protein. C-Reactive Protein (CRP), the prototypic marker of inflammation, is a cardiovascular risk marker and may promote atherogenesis.
钙蛋白酶-1的内部淬灭肽底物具有最佳的裂解基序,位于易裂键两侧,干扰人中性粒细胞膜相关的氧化代谢。
An internally quenched peptide substrate for calpain-1 with optimal cleavage motifs flanking the scissile bond, interferes with the membrane-associated oxidative metabolism in human neutrophils.
化学预防肽是有助于预防疾病(例如癌症或糖尿病)的发作或发展的肽。这些肽可以源自天然来源,例如大豆或牛奶,也可以来自肽模拟物的设计,也可以源自使用合成肽进行的肽筛选。据认为,这些肽中的某些可以充当细胞周期的调节剂,其调节使细胞通过复制周期前进所需的蛋白质的产生和功能。另外,现在有越来越多的证据表明特定的饮食模式,食物和饮料以及其他饮食物质可以而且确实可以预防癌症。越来越多的流行病学研究表明,食物,营养和身体活动在预防和改变癌症过程中很重要。包括植物蛋白酶抑制剂,乳铁蛋白,乳铁蛋白,凝集素和lunasin在内的不同类型的食物蛋白和多肽似乎起着化学预防剂的作用。如今,蛋白质和多肽被认为是一组营养保健品,在预防癌症的不同阶段(包括起始,促进和进展)方面显示出潜力。此外,已经发现在植物中发现的一些蛋白酶抑制剂,例如豆类和大豆,是有效的癌发生抑制剂。致癌作用是引发和促进癌症的过程。 Bowman-Birk抑制剂和Kunitz胰蛋白酶抑制剂就在其中。目前,这些化合物在致癌作用中的生物学功能主要归因于抑制癌细胞的侵袭和转移,但是,其作用机理仍不完全清楚,需要进一步研究以充分阐明它们。
Definition
C-reactive protein (CRP) is an acute phase protein. It is phylogenetically ancient and - with serum amyloid P - belongs to proteins named as "pentraxin".
Discovery
CRP was discovered in Oswald Avery's laboratory during the course of studies of patients with Streptococcus pneumoniae infection. Sera obtained from these patients during the early, acute phase of the illness were found to contain a protein that could precipitate the “C” polysaccharide derived from the pneumococcal cell wall1.
Structural Characteristics
CRP belongs to the pentraxin family of calcium-dependent ligand-binding plasma proteins, the other member of which in humans is serum amyloid P component (SAP). The human CRP molecule (Mr 115,135) is composed of five identical nonglycosylated polypeptide subunits (Mr 23,027), each containing 206 amino acid residues. The protomers are noncovalently associated in an annular configuration with cyclic pentameric symmetry. Each protomer has the characteristic "lectin fold," composed of a two-layered ß sheet with flattened jellyroll topology. The ligand-binding site, composed of loops with two calcium ions bound 4 Å apart by protein side-chains, is located on the concave face. The other face carries a single a helix2.
Mode of Action
Human CRP binds with highest affinity to phosphocholine residues, but it also binds to a variety of other autologous and extrinsic ligands, and it aggregates or precipitates the cellular, particulate, or molecular structures bearing these ligands. Autologous ligands include native and modified plasma lipoproteins, damaged cell membranes, a number of different phospholipids and related compounds, small nuclear ribonucleoprotein particles, and apoptotic cells. Extrinsic ligands include many glycan, phospholipid, and other constituents of microorganisms, such as capsular and somatic components of bacteria, fungi, and parasites, as well as plant products. When aggregated or bound to macromolecular ligands, human CRP is recognized by C1q and potently activates the classical complement pathway, engaging C3, the main adhesion molecule of the complement system, and the terminal membrane attack complex, C5–C9. Bound CRP may also provide secondary binding sites for factor H and thereby regulate alternative-pathway amplification and C5 convertases3.
Functions
CRP, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events: The metabolic syndrome describes a high-risk population having 3 or more of the following clinical characteristics: upper-body obesity, hypertriglyceridemia, low HDL, hypertension, and abnormal glucose. All of these attributes, however, are associated with increased levels of CRP. In a study, the interrelationships between CRP, the metabolic syndrome, and incident cardiovascular events was evaluated among 14,719 apparently healthy women who were followed up for an 8-year period for myocardial infarction, stroke, coronary revascularization, or cardiovascular death; 24% of the cohort had the metabolic syndrome at study entry. It was found that, at baseline, median CRP levels for those with 0, 1, 2, 3, 4, or 5 characteristics of the metabolic syndrome were 0.68, 1.09, 1.93, 3.01, 3.88, and 5.75 mg/L, respectively (Ptrend <0.0001). Over the 8-year follow-up, cardiovascular event-free survival rates based on CRP levels above or below 3.0 mg/L were similar to survival rates based on having 3 or more characteristics of the metabolic syndrome. At all levels of severity of the metabolic syndrome, however, CRP added prognostic information on subsequent risk. Additive effects for CRP were also observed for those with 4 or 5 characteristics of the metabolic syndrome. These prospective data suggest that measurement of CRP adds clinically important prognostic information to the metabolic syndrome4.
CRP and the pathogenesis of atherosclerosis: The CRP binds to lipids, especially lecithin (phosphatidyl choline), and to plasma lipoproteins, and the first suggestion of a possible relationship to atherosclerosis came when it was demonstrated that aggregated, but not native, non-aggregated, CRP selectively bound just LDL and some VLDL from whole serum. However, native CRP does bind to partially degraded, so-called modified LDL, as it is found in atheromatous plaques, and to oxidized LDL. Furthermore CRP is present in most such plaques examined ex vivo. This CRP could contribute to complement activation and thus inflammation in the plaques, and there is experimental evidence supporting a possible role of complement in atherogenesis. CRP has also been reported to stimulate tissue factor production by peripheral blood monocytes and could thereby have important pro-coagulant effects5.
Targeting CRP for the treatment of cardiovascular disease: A study, reported the design, synthesis and efficacy of 1,6-bis(phosphocholine)-hexane as a specific small-molecule inhibitor of CRP. Five molecules of palindromic compound are bound by two pentameric CRP molecules, crosslinking and occluding the ligand-binding B-face of CRP and blocking its functions. Administration of 1,6-bis(phosphocholine)-hexane to rats undergoing acute myocardial infarction abrogated the increase in infarct size and cardiac dysfunction produced by injection of human CRP. Therapeutic inhibition of CRP is thus a promising new approach to cardioprotection in acute myocardial infarction. Potential wider applications include other inflammatory, infective and tissue-damaging conditions characterized by increased CRP production, in which binding of CRP to exposed ligands in damaged cells may lead to complement-mediated exacerbation of tissue injury6.
References
Tillett WS, Francis Jr T (1930). Serological reactions in pneumonia with a nonprotein somatic fraction of pneumococcus. J Exp Med., 52:561–585.
Thompson D, Pepys MB, Wood SP (1999). The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure, 7:169-177.
Pepys MB, Hirschfield GM (2003). C-reactive protein: a critical update. J. Clin Invest., 111(12):1805-1812.
Ridker PM, Buring JE, Cook NR, Rifai N (2003). C-Reactive Protein, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events (An 8-Year Follow-Up of 14 719 Initially Healthy American Women). Circulation, 107(3):391-397.
Pepys MB, Hirschfield GM (2003). C-reactive protein and cardiovascular disease: new insights from an old molecule. Q. J. Med., 96:793-807.
Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, Hawkins PN, Myers RM, Smith MD, Polara A, Cobb AJ, Ley SV, Aquilina JA, Robinson CV, Sharif I, Gray GA, Sabin CA, Jenvey MC, Kolstoe SE, Thompson D, Wood SP (2006). Targeting C-reactive protein for the treatment of cardiovascular disease. Nature, 440(7088):1217-1221.
| DOI | 名称 | |
|---|---|---|
| 10.1016/j.atherosclerosis.2008.05.060 | C-reactive protein stimulates superoxide anion release and tissue factor activity in vivo | 下载 |
多肽H2N-Lys-Pro-Gln-Leu-Trp-Pro-COOH的合成步骤:
1、合成CTC树脂:称取0.86g CTC Resin(如初始取代度约为0.35mmol/g)和0.36mmol Fmoc-Pro-OH于反应器中,加入适量DCM溶解氨基酸(需要注意,此时CTC树脂体积会增大好几倍,避免DCM溶液过少),再加入0.9mmol DIPEA(Mw:129.1,d:0.740g/ml),反应2-3小时后,可不抽滤溶液,直接加入1ml的HPLC级甲醇,封端半小时。依次用DMF洗涤2次,甲醇洗涤1次,DCM洗涤一次,甲醇洗涤一次,DCM洗涤一次,DMF洗涤2次(这里使用甲醇和DCM交替洗涤,是为了更好地去除其他溶质,有利于后续反应)。得到 Fmoc-Pro-CTC Resin。结构图如下:

2、脱Fmoc:加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Pro-CTC Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:

3、缩合:取0.9mmol Fmoc-Trp(Boc)-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入1.81mmol DIPEA,0.86mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Trp(Boc)-Pro-CTC Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:
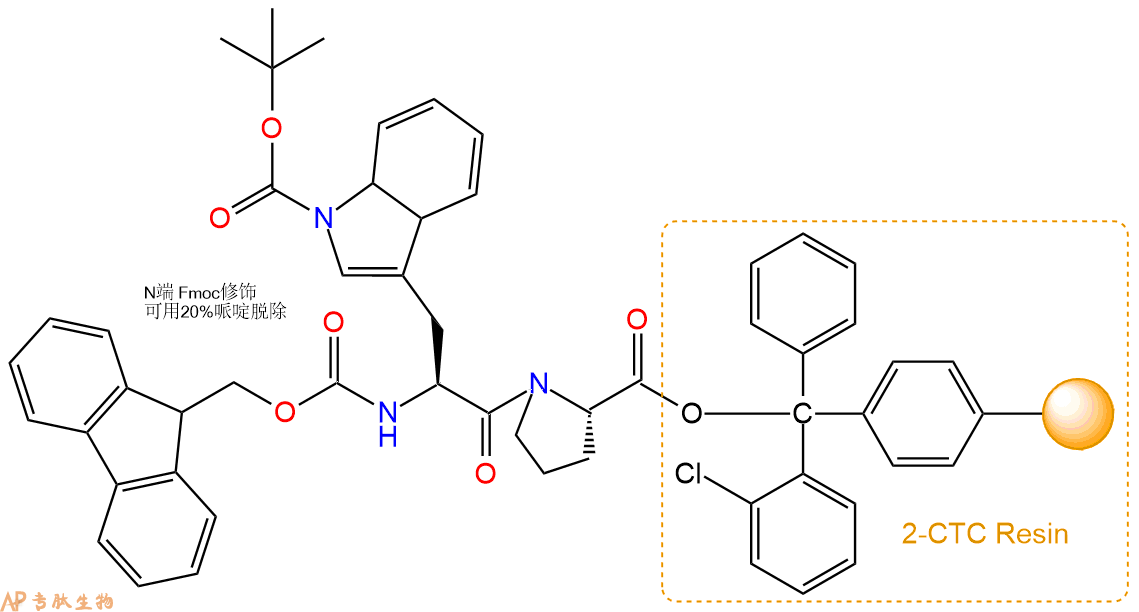
4、依次循环步骤二、步骤三,依次得到
H2N-Trp(Boc)-Pro-CTC Resin
Fmoc-Leu-Trp(Boc)-Pro-CTC Resin
H2N-Leu-Trp(Boc)-Pro-CTC Resin
Fmoc-Gln(Trt)-Leu-Trp(Boc)-Pro-CTC Resin
H2N-Gln(Trt)-Leu-Trp(Boc)-Pro-CTC Resin
Fmoc-Pro-Gln(Trt)-Leu-Trp(Boc)-Pro-CTC Resin
H2N-Pro-Gln(Trt)-Leu-Trp(Boc)-Pro-CTC Resin
Fmoc-Lys(Boc)-Pro-Gln(Trt)-Leu-Trp(Boc)-Pro-CTC Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Lys(Boc)-Pro-Gln(Trt)-Leu-Trp(Boc)-Pro-CTC Resin,结构如下:
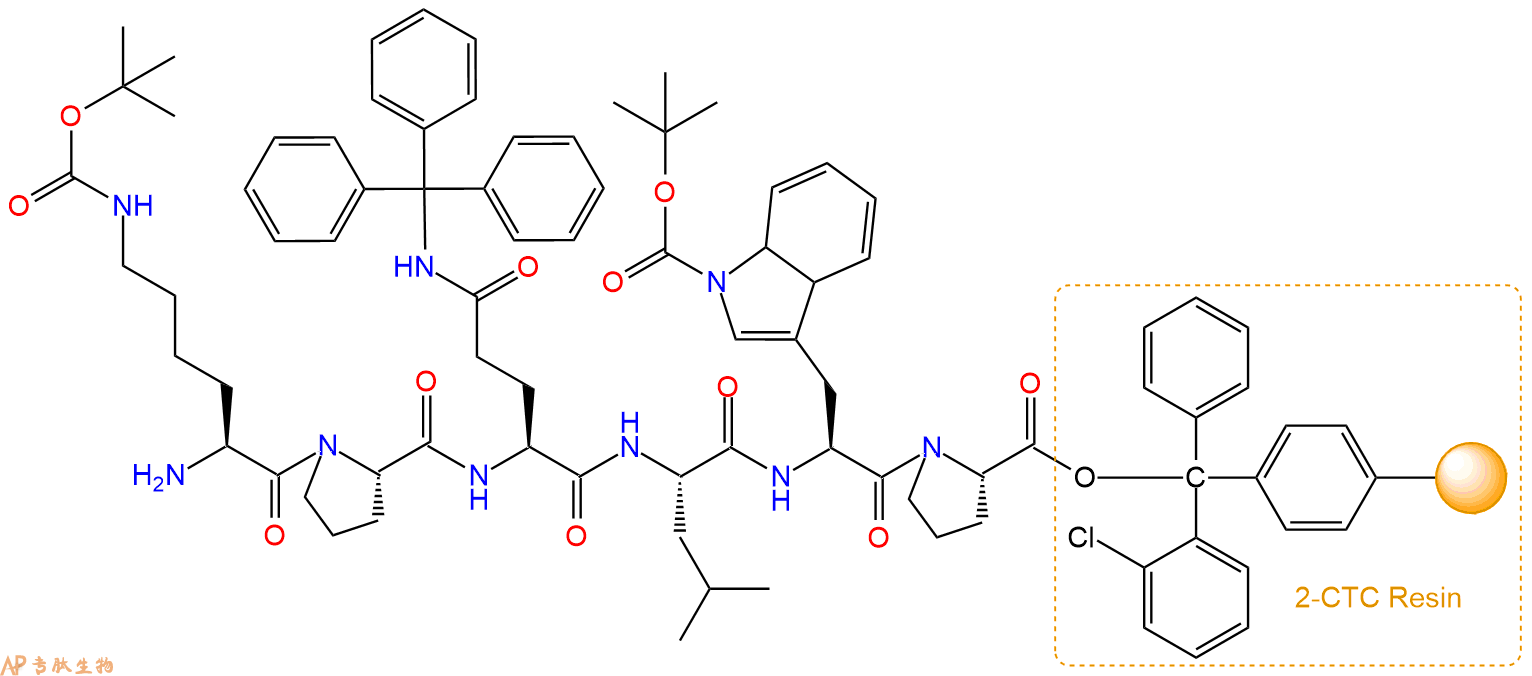
5、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品H2N-Lys-Pro-Gln-Leu-Trp-Pro-COOH。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%、TFA:H2O=97.5%:2.5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





