刘犇教授课题组在INORGANIC CHEMISTRY发表研究论文
"Dual-Template"-Directed Synthesis of Bowl-Shaped Mesoporous Platinum Nanostructures
Lv, H (Lv, Hao)[ 1 ] ; Chen, X (Chen, Xin)[ 2 ] ; Fu, C (Fu, Cheng)[ 1 ] ; She, PL (She, Peiliang)[ 1 ] ; Xu, DD (Xu, Dongdong)[ 1 ] ; Liu, B (Liu, Ben)[ 1 ]*(刘犇)
[ 1 ] Nanjing Normal Univ, Sch Chem & Mat Sci, Jiangsu Collaborat Innovat Ctr Biomed Funct Mat, Jiangsu Key Lab New Power Batteries, Nanjing 210023, Jiangsu, Peoples R China
[ 2 ] ME Genom Inc, Software Ind Base, Shenzhen 518000, Peoples R China
INORGANIC CHEMISTRY,201908,58(16),11195-11201
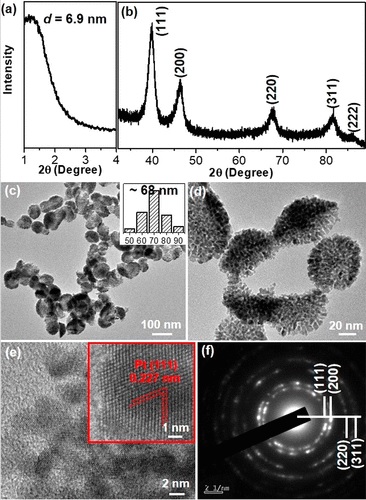
Asymmetric bowl-shaped metals make up an important class of nanostructured materials that exhibit great potentials in catalysis, energy storage, and biomedical applications. Introducing the mesopores within the framework of bowl-shaped metals would further increase the surface area and thus increase the utilization efficiency of metals and the availability of the (electro)catalytically active sites. In this work, a one-pot surfactant-templated aqueous synthesis is developed to fabricate nanosized asymmetric platinum (Pt) bowl-shaped mesoporous nanospheres (BMSs) with good purity and uniformity. Amphiphilic dioctadecyldimethylammonium chloride acts as the "dual-template" surfactant that drives the anisotropic nucleation and growth of mesoporous Pt islands with cylinder micelles along the curved surface of the vesicles, resulting in the formation of the Pt BMSs with a bowl-shaped morphology and mesoporous structure. The diameter of the Pt BMSs can also be tailored by changing the reduction kinetics during the synthesis. More interestingly, the BMSs are able to interconnect into a more sophisticated structure of one-dimensional nanochains by increasing the K2PtCl4 amount added. This novel synthetic protocol brings a higher hierarchy into asymmetric bowl-shaped metals, rendering the metal BMSs with more accessible (electro)catalytically active sites. Because of the unique morphology and structure, the Pt BMSs show enhanced electrocatalytic activity and stability toward the hydrogen evolution reaction with respect to a commercial Pt/C catalyst.
文章链接:
https://pubs.acs.org/doi/10.1021/acs.inorgchem.9b01794
版权与免责声明:本网页的内容由收集互联网上公开发布的信息整理获得。目的在于传递信息及分享,并不意味着赞同其观点或证实其真实性,也不构成其他建议。仅提供交流平台,不为其版权负责。如涉及侵权,请联系我们及时修改或删除。邮箱:sales@allpeptide.com